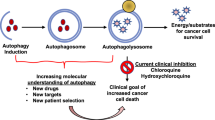
Key Points
-
Autophagy is a process that describes the degradation and recycling of proteins and intracellular components in response to starvation or stress.
-
At the early stage of tumour development, autophagy functions as a tumour suppressor. Expression of beclin 1 (BECN1), a mammalian orthologue of the yeast autophagy-related gene Atg6, reduces tumorigenic capacity through induction of autophagy. Mice that are Becn1+/− display a remarkable increase in the incidence of lung cancer, hepatocellular carcinoma and lymphoma.
-
At advanced stages of tumour development, autophagy promotes tumour progression. The tumour cells that are located in the central area of the tumour mass undergo autophagy to survive low-oxygen and low-nutrient conditions.
-
Autophagy protects some cancer cells against anticancer treatments by blocking the apoptotic pathway ('protective autophagy'). By contrast, other cancer cells undergo autophagic cell death after cancer therapies.
-
Autophagy is induced mainly through the phosphatidylinositol 3-phosphate kinase (PI3K)–AKT–mTOR (mammalian target of rapamycin) signalling pathway.
-
Manipulation of autophagy has the potential to improve anticancer therapeutics. When tumour cells induce protective autophagy, inhibition of autophagy could sensitize tumour cells to the treatment by activating apoptosis. On the other hand, induction of autophagic cell death can also have a therapeutic value.
Abstract
Autophagy is a process in which subcellular membranes undergo dynamic morphological changes that lead to the degradation of cellular proteins and cytoplasmic organelles. This process is an important cellular response to stress or starvation. Many studies have shed light on the importance of autophagy in cancer, but it is still unclear whether autophagy suppresses tumorigenesis or provides cancer cells with a rescue mechanism under unfavourable conditions. What is the present state of our knowledge about the role of autophagy in cancer development, and in response to therapy? And how can the autophagic process be manipulated to improve anticancer therapeutics?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Klionsky, D. J. & Emr, S. D. Autophagy as a regulated pathway of cellular degradation. Science 290, 1717–1721 (2000). Comprehensive review of autophagic processes.
Levine, B. & Klionsky, D. J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 (2004). Comprehensive review of autophagy in eukaryotic development.
Meijer, A. J. & Codogno, P. Regulation and role of autophagy in mammalian cells. Int. J. Biochem. Cell Biol. 36, 2445–2462 (2004). Comprehensive review of the autophagic process in mammalian cells.
Larsen, K. E. & Sulzer, D. Autophagy in neurons: a review. Histol. Histopathol. 17, 897–908 (2002).
Nishino, I. Autophagic vacuolar myopathies. Curr. Neurol. Neurosci. Rep. 3, 64–69 (2003).
Ogier-Denis, E. & Codogno, P. Autophagy: a barrier or an adaptive response to cancer. Biochim. Biophys. Acta 1603, 113–128 (2003).
Gozuacik, D. & Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23, 2891–2906 (2004). Reference 7, together with reference 6, provide comprehensive reviews of autophagy in cancer.
Gunn, J. M., Clark, M. G., Knowles, S. E., Hopgood, M. F. & Ballard, F. J. Reduced rates of proteolysis in transformed cells. Nature 266, 58–60 (1977).
Kisen, G. O. et al. Reduced autophagic activity in primary rat hepatocellular carcinoma and ascites hepatoma cells. Carcinogenesis 14, 2501–2505 (1993).
Kirkegaard, K., Taylor, M. P. & Jackson, W. T. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nature Rev. Microbiol. 2, 301–314 (2004).
Otsuka, H. & Moskowitz, M. Differences in the rates of protein degradation in untransformed and transformed cell lines. Exp. Cell Res. 112, 127–35 (1978).
Schwarze, P. E. & Seglen, P. O. Reduced autophagic activity, improved protein balance and enhanced in vitro survival of hepatocytes isolated from carcinogen-treated rats. Exp. Cell Res. 157, 15–28 (1985).
Lee, H. K., Jones, R. T., Myers, R. A. & Marzella, L. Regulation of protein degradation in normal and transformed human bronchial epithelial cells in culture. Arch. Biochem. Biophys. 296, 271–278 (1992).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 (1999). First demonstration of the relationship between autophagy-associated BECN1 and tumorigenicity in breast and other cancers.
Liang, X. H. et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel BCL-2-interacting protein. J. Virol. 72, 8586–96 (1998).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003). First evidence of tumour development because of a deficiency of BECN1.
Yue, Z., Jin, S., Yang, C., Levine, A. J. & Heintz, N. beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl Acad. Sci. USA 100, 15077–15082 (2003). Reference 17, together with reference 16, provide the first demonstrations of the role of BECN1 as a tumour suppressor.
Cuervo, A. M. Autophagy: in sickness and in health. Trends Cell Biol. 14, 70–77 (2004).
Edinger, A. L. & Thompson, C. B. Defective autophagy leads to cancer. Cancer Cell 4, 422–424 (2003).
Ogier-Denis, E., Houri, J. J., Bauvy, C. & Codogno, P. Guanine nucleotide exchange on heterotrimeric GI3 protein controls autophagic sequestration in HT-29 cells. J. Biol. Chem. 271, 28593–28600 (1996).
Liang, X. H., Yu, J., Brown, K. & Levine, B. Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res. 61, 3443–3449 (2001).
Proikas-Cezanne, T. et al. WIPI-1α (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene 23, 9314–9325 (2004).
Susan, P. P. & Dunn, W. A. Jr. Starvation-induced lysosomal degradation of aldolase B requires glutamine 111 in a signal sequence for chaperone-mediated transport. J. Cell. Physiol. 187, 48–58 (2001).
Ito, H., Daido, S., Kanzawa, T., Kondo, S. & Kondo, Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int. J. Oncol. 26, 1401–1410 (2005).
Furuta, S., Hidaka, E., Ogata, A., Yokota, S. & Kamata, T. RAS is involved in the negative control of autophagy through the class I PI3-kinase. Oncogene 23, 3898–3904 (2004).
Izuishi, K., Kato, K., Ogura, T., Kinoshita, T. & Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res. 60, 6201–6207 (2000).
Clarke, P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol (Berl). 181, 195–213 (1990). Renews interest in the role of autophagy during cell death.
Bursch, W. et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis 17, 1595–1607 (1996).
Scarlatti, F. et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of Beclin 1. J. Biol. Chem. 279, 18384–18391 (2004).
Kanzawa, T. et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 11, 448–457 (2004). First investigation to find that inhibition of autophagy at different stages causes distinct outcomes.
Paglin, S. et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 61, 439–444 (2001). First investigation to show the feasibility of treating cancer cells by autophagy inhibition.
Yao, K. C. et al. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J. Neurosurg. 98, 378–384 (2003).
Shao, Y., Gao, Z., Marks, P. A. & Jiang, X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA, 101, 18030–18035 (2004).
Komata, T. et al. Mild heat shock induces autophagic growth arrest, but not apoptosis in U251-MG and U87-MG human malignant glioma cells. J. Neurooncol. 68, 101–111 (2004).
Kanzawa, T., Kondo, Y., Ito, H., Kondo, S., and Germano, I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 63, 2103–2108 (2003). Provides evidence that bafilomycin A 1 increases the antitumour effect of arsenic trioxide by inhibiting autophagy.
Kanzawa, T. et al. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene 24, 980–991 (2005).
Opipari, A. W. Jr. et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 64, 696–703 (2004).
Ellington, A. A., Berhow, M. & Singletary, K. W. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis 26, 159–167 (2005).
Takeuchi, H. et al. Augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-phosphate kinase/protein kinase B inhibitors. Cancer Res. 65, 3336–3346 (2005).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Shintani, T. & Klionsky, D. J. Autophagy in health and disease: a double-edged sword. Science 306, 990–995 (2004). Reviews a role of autophagy in health and disease.
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000). Reviews the function of TOR and mTOR as central regulators for cell growth.
Gingras, A. C., Raught, B. & Sonenberg, N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15, 807–826 (2001). Review shows that mTOR is involved in the regulation of translation initiation.
Wang, C. W. & Klionsky, D. J. The molecular mechanism of autophagy. Mol. Med. 3–4, 65–76 (2003).
Petiot, A., Ogier-Denis, E., Blommaart, E. F., Meijer, A. J. & Codogno, P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998 (2000).
Arico, S. et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 276, 35243–35246 (2001).
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997).
Kihara, A., Kabeya, Y., Ohsumi, Y. & Yoshimori, T. Beclin–phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2, 330–335 (2001).
Brown, W. J., DeWald, D. B., Emr, S. D., Plutner, H. & Balch, W. E. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol. 130, 781–796 (1995).
Inbal, B., Bialik, S., Sabanay, I., Shani, G. & Kimchi, A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J. Cell Biol. 157, 455–468 (2002). Demonstrates the induction of autophagic cell death by DAPK and DRP1.
Saeki, K. et al. BCL-2 down-regulation causes autophagy in a caspase-independent manner in human leukemic HL60 cells. Cell Death Differ. 7, 1263–1269 (2000).
Vande Velde, C. et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol. Cell. Biol. 20, 5454–5468 (2000). Demonstrates the involvement of BNIP3 in autophagic cell death.
Yanagisawa, H., Miyashita, T., Nakano, Y. & Yamamoto, D. HSPIN1, a transmembrane protein interacting with BCL-2/BCL-XL, induces a caspase-independent autophagic cell death. Cell Death Differ., 10, 798–807 (2003).
Ogier-Denis, E., Pattingre, S., El Benna, J. & Codogno, P. ERK1/2-dependent phosphorylation of Gα-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J. Biol. Chem. 275, 39090–39095 (2000).
Inbal, B. et al. DAP kinase links the control of apoptosis to metastasis. Nature 390, 180–184 (1997).
Daido, S. et al. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 64, 4286–4293 (2004).
Shimizu, S. et al. Role of BCL-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cell Biol. 6, 1221–1228 (2004).
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005).
Su, B. & Karin, M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 8, 402–411 (1996).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Alva, A. S., Gultekin, S. H. & Baehrecke, E. H. Autophagy in human tumors: cell survival or death? Cell Death Differ. 11, 1046–1048 (2004).
Bursch, W., Ellinger, A., Gerner, C., Frohwein, U. & Schulte-Hermann, R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others? Ann. NY Acad. Sci. 926, 1–12 (2000).
Bursch, W. et al. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J. Cell Sci. 113, 1189–1198 (2000).
Bursch, W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 8, 569–581 (2001).
Yu, L. et al. Regulation of an ATG7–Beclin 1 program of autophagic cell death by caspase-8. Science 304, 1500–1502 (2004). Demonstrates the direct interaction between autophagy and apoptosis.
Boya, P. et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol., 25, 1025–1040 (2005).
Bauvy, C., Gane, P., Arico, S., Codogno, P. & Ogier-Denis, E. Autophagy delays sulindac sulfide-induced apoptosis in the human intestinal colon cancer cell line HT-29. Exp. Cell Res. 268, 139–149 (2001).
Pelicano, H. et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 278, 37832–37839 (2003).
Piacentini, M., Evangelisti, C., Mastroberardino, P. G., Nardacci, R. & Kroemer, G. Does prothymosin-α act as molecular switch between apoptosis and autophagy? Cell Death Differ. 10, 937–939 (2003).
Yamamoto, A. et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 (1998).
Cheney, I. W. et al. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 58, 2331–2334 (1998).
Davies, M. A. et al. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin. Cancer Res. 8, 1904–1914 (2002).
Chan, S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br. J. Cancer 91, 1420–1424 (2004).
Eshleman, J. S. et al. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 62, 7291–7297 (2002).
Mondesire, W. H. et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin. Cancer Res. 10, 7031–7042 (2004).
Stephan, S. et al. Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clin. Cancer Res. 10, 6993–7000 (2004).
Kim, J. & Klionsky, D. J. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Ann. Rev. Biochem. 69, 303–342 (2000).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast APG8P, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Mizushima, N. et al. Dissection of autophagosome formation using APG5-deficient mouse embryonic stem cells. J. Cell Biol. 152, 657–668 (2001). First demonstration of the de novo synthesis of the autophagosome membrane.
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 (2004).
Dunn, W. A. Jr. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J. Cell Biol. 110, 1923–1933 (1990).
Yokota, S., Himeno, M., Roth, J., Brada, D. & Kato, K. Formation of autophagosomes during degradation of excess peroxisomes induced by di-(2-ethylhexyl)phthalate treatment. II. Immunocytochemical analysis of early and late autophagosomes. Eur. J. Cell Biol. 62, 372–383 (1993).
Stromhaug, P. E., Berg, T. O., Fengsrud, M. & Seglen, P. O. Purification and characterization of autophagosomes from rat hepatocytes. Biochem. J. 335, 217–224 (1998).
Noda, T., Suzuki, K. & Ohsumi, Y. Yeast autophagosomes: de novo formation of a membrane structure. Trends Cell Biol. 12, 231–25 (2002).
Reunanen, H., Marttinen, M. & Hirsimaki, P. Effects of griseofulvin and nocodazole on the accumulation of autophagic vacuoles in Ehrlich ascites tumor cells. Exp. Mol. Pathol. 48, 97–102 (1988).
Punnonen, E. L. & Reunanen, H. Effects of vinblastine, leucine, and histidine, and 3-methyladenine on autophagy in Ehrlich ascites cells. Exp. Mol. Pathol. 52, 87–97 (1990).
Acknowledgements
We thank Akitsugu Yamamoto (Nagahama Institute of Bio-Science and Technology, Nagahama, Japan) and Noboru Mizushima (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) for their critical review, and Karen F. Phillips, ELS, for editing the manuscript. This work was supported in part by National Cancer Institute grants (S.K.), in part by a start-up fund (S.K.), Institutional Research grant (Y.K.), and a Cancer Center Support grant/Shared Resources from The University of Texas M. D. Anderson Cancer Center, and in part by a generous donation from the Anthony D. Bullock III Foundation (Y.K., R.S. and S.K.). We apologize to colleagues whose works on cancer-related autophagy have not been cited owing to space limitation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
Glossary
- AUTOPHAGY
-
Lysosome-mediated degradation of proteins and cellular organelles. Autophagic cell death is referred to as type II programmed cell death.
- AUTOPHAGOSOME
-
A membrane structure, formed inside cells during the process of autophagy, which sequesters cellular proteins and cytoplasmic organelles.
- AUTOLYSOSOME
-
A membrane structure made by the fusion of an autophagosome and a lysosome.
- APOPTOSIS
-
Referred to as type I programmed cell death. Characterized by a particular pattern of morphological changes, such as chromatin condensation or fragmentation.
- TRANS-GOLGI NETWORK
-
The last three cisternae of the Golgi apparatus, which is made up of seven cisternae altogether. This is the exit compartment for newly made proteins that are on the way to their destinations.
- PROGRAMMED CELL DEATH
-
An active cellular process that results in cell death. It takes place during normal development and in response to physiological damage such as that caused by cancer treatments.
Rights and permissions
About this article
Cite this article
Kondo, Y., Kanzawa, T., Sawaya, R. et al. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5, 726–734 (2005). https://doi.org/10.1038/nrc1692
Issue Date:
DOI: https://doi.org/10.1038/nrc1692
This article is cited by
-
The Mediator Complex Subunit 12 (MED-12) Gene and Uterine Fibroids: a Systematic Review
Reproductive Sciences (2024)
-
The role of m6A methylation in therapy resistance in cancer
Molecular Cancer (2023)
-
Inactivation of ZSCAN18 by promoter hypermethylation drives the proliferation via attenuating TP53INP2-mediated autophagy in gastric cancer cells
Clinical Epigenetics (2023)
-
From signalling pathways to targeted therapies: unravelling glioblastoma’s secrets and harnessing two decades of progress
Signal Transduction and Targeted Therapy (2023)
-
BCL2L13 promotes mitophagy through DNM1L-mediated mitochondrial fission in glioblastoma
Cell Death & Disease (2023)