Key Points
-
Cellular senescence is a mechanism that blocks the proliferation of primary, and in some cases premalignant and malignant, cells. It can be activated by a plethora of stress conditions, including oncogene activation, loss of tumour suppressors or critical shortening of telomeres.
-
Oncogene-induced senescence has recently been recognized as a tumour-suppressive mechanism in vivo, in human lesions and in several mouse tumour models.
-
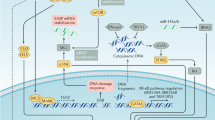
Recent evidence suggests that the induction of senescence requires several secreted factors, including members of Wnt, insulin, transforming growth factor-β, plasmin, interleukin and possibly also interferon signalling cascades. We term these collectively the senescence-messaging secretome (SMS).
-
The SMS and its signalling cascades may converge first at the level of several plasma membrane signalling receptors.
-
The use of secreted factors in senescence could provide a selective advantage to an organism, as it allows for communication between senescent cells and their microenvironment.
-
Counterintuitively, senescent cells may contribute to tumorigenesis by virtue of the SMS, which can cause stromal components to senesce, thereby establishing a pro-mitogenic loop.
Abstract
Oncogene-induced cellular senescence constitutes a strong anti-proliferative response, which can be set in motion following either oncogene activation or loss of tumour suppressor signalling. It serves to limit the expansion of early neoplastic cells and as such is a potent cancer-protective response to oncogenic events. Recently emerging evidence points to a crucial role in oncogene-induced cellular senescence for the 'senescence-messaging secretome' or SMS, setting the stage for cross-talk between senescent cells and their environment. How are such signals integrated into a coordinated response and what are the implications of this unexpected finding?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
13 January 2009
In the version of this article initially published online, table 1 read "ERK" in place of "phosphoERK" in row 5 column 4, and "replication" in place of "replicative senescence" in row 6 column 3. Additionally, the fourth paragraph under "Interleukins" read "paracrine promoting functions" in place of "paracrine tumour-promoting functions". The errors have been corrected for the print, HTML and PDF versions of the article
References
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997). A seminal observation of OIS.
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965). A landmark paper about the mortality of primary cells.
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Adams, P. D. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene 397, 84–93 (2007).
Deng, Y., Chan, S. & Chang, S. Telomere dysfunction and tumour suppression: the senescence connection. Nature Rev. Cancer 8, 450–458 (2008).
Ben-Porath, I. & Weinberg, R. A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976 (2005).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005). A review about positive, but also adverse, effects of senescence on tumorigenesis, describing the potential role of a senescence-associated secretome.
Bennett, D. C. Human melanocyte senescence and melanoma susceptibility genes. Oncogene 22, 3063–3069 (2003).
Prieur, A. & Peeper, D. S. Cellular senescence in vivo: a barrier to tumorigenesis. Curr. Opin. Cell Biol. 20, 150–155 (2008).
Mooi, W. J. & Peeper, D. S. Oncogene-induced cell senescence — halting on the road to cancer. N. Engl. J. Med. 355, 1037–1046 (2006).
D'adda Di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nature Rev. Cancer 8, 512–522 (2008).
Finkel, T., Serrano, M. & Blasco, M. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
Cristofalo, V. J. & Pignolo, R. J. Molecular markers of senescence in fibroblast-like cultures. Exp. Gerontol. 31, 111–123 (1996).
Goldstein, S., Moerman, E. J., Jones, R. A. & Baxter, R. C. Insulin-like growth factor binding protein 3 accumulates to high levels in culture medium of senescent and quiescent human fibroblasts. Proc. Natl Acad. Sci. USA 88, 9680–9684 (1991).
Ferber, A. et al. Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J. Biol. Chem. 268, 17883–17888 (1993).
Goldstein, S., Moerman, E. J., Fujii, S. & Sobel, B. E. Overexpression of plasminogen activator inhibitor type-1 in senescent fibroblasts from normal subjects and those with Werner syndrome. J. Cell Physiol. 161, 571–579 (1994).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nature Rev. Mol. Cell Biol. 8, 729–740 (2007).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008). Both these papers describe the essential role of cytokines and their receptors in cellular senescence.
Larsson, O., Girnita, A. & Girnita, L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br. J. Cancer 92, 2097–2101 (2005).
Russell, S. J. & Kahn, C. R. Endocrine regulation of ageing. Nature Rev. Mol. Cell Biol. 8, 681–691 (2007).
Firth, S. M. & Baxter, R. C. Cellular actions of the insulin-like growth factor binding proteins. Endocrine Rev. 23, 824–854 (2002).
Kim, K. S. et al. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell 6, 535–545 (2007).
Kim, K. S. et al. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol. Biol. Cell 18, 4543–4552 (2007).
Muck, C., Micutkova, L., Zwerschke, W. & Jansen-Durr, P. Role of insulin-like growth factor binding protein-3 in human umbilical vein endothelial cell senescence. Rejuvenation Res. 11, 449–453 (2008).
Sprenger, C. C., Vail, M. E., Evans, K., Simurdak, J. & Plymate, S. R. Over-expression of insulin-like growth factor binding protein-related protein-1(IGFBP-rP1/mac25) in the M12 prostate cancer cell line alters tumor growth by a delay in G1 and cyclin A associated apoptosis. Oncogene 21, 140–147 (2002).
Wilson, H. M., Birnbaum, R. S., Poot, M., Quinn, L. S. & Swisshelm, K. Insulin-like growth factor binding protein-related protein 1 inhibits proliferation of MCF-7 breast cancer cells via a senescence-like mechanism. Cell Growth Differ. 13, 205–213 (2002).
Wajapeyee, N., Serra, R. W., Zhu, X., Mahalingam, M. & Green, M. R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374 (2008). References 24, 25 and 29 show the dependence of senescence on IGFBP3, IGFBP5 and IGFBP7.
Pollock, P. et al. High frequency of BRAF mutations in nevi. Nature Genet. 33, 19–20 (2003).
Michaloglou, C., Vredeveld, L. C., Mooi, W. J. & Peeper, D. S. BRAFE600 in benign and malignant human tumours. Oncogene 27, 877–895 (2008).
Courtois-Cox, S. et al. A negative feedback signalling network underlies oncogene-induced senescence. Cancer Cell 10, 459–472 (2006).
Denoyelle, C. et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nature Cell Biol. 8, 1053–1063 (2006).
Gray-Schopfer, V. C. et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br. J. Cancer 95, 496–505 (2006).
Chakravarthy, M. V., Abraha, T. W., Schwartz, R. J., Fiorotto, M. L. & Booth, F. W. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/Akt signaling pathway. J. Biol. Chem. 275, 35942–35952 (2000).
Park, G. H. & Buetow, D. E. Genes for insulin-like growth factors I and II are expressed in senescent rat tissues. Gerontology 37, 310–316 (1991).
Fu, V. X. et al. A loss of insulin-like growth factor-2 imprinting is modulated by CCCTC-binding factor down-regulation at senescence in human epithelial cells. J. Biol. Chem. 279, 52218–52226 (2004).
Hernandez, L., Kozlov, S., Piras, G. & Stewart, C. L. Paternal and maternal genomes confer opposite effects on proliferation, cell-cycle length, senescence, and tumor formation. Proc. Natl Acad. Sci. USA 100, 13344–13349 (2003).
Ghosh, P., Dahms, N. M. & Kornfeld, S. Mannose 6-phosphate receptors: new twists in the tale. Nature Rev. Mol. Cell Biol. 4, 202–212 (2003).
Hung., P. et al. Insulin-like growth factor binding protein-5 (IGFBP-5) suppresses the tumourigenesis of head and neck squamous cell carcinoma. J. Pathol. 214, 368–376 (2008).
Hanafusa, T. et al. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 176, 149–158 (2002).
Tomii, K. et al. Aberrant promoter methylation of insulin-like growth factor binding protein-3 gene in human cancers. Int. J. Cancer 120, 566–573 (2007).
Collado, M. & Serrano, M. The power and the promise of oncogene-induced senescence markers. Nature Rev. Cancer 6, 472–476 (2006).
Kortlever, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature Cell Biol. 8, 877–884 (2006). This articles demonstrates a causal link between PAI1 and cellular senescence.
Kortlever, R. M. & Bernards, R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle 5, 2697–2703 (2006).
Dass, K., Ahmad, A., Azmi, A. S., Sarkar, S. H. & Sarkar, F. H. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 34, 122–136 (2008).
Tremain, R. et al. Defects in TGF-beta signaling overcome senescence of mouse keratinocytes expressing v-Ha-ras. Oncogene 19, 1698–1709 (2000). The first paper to describe a role for a secreted factor, that is, TGFβ in senescence.
Massagué, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Kortlever, R. M., Nijwening, J. H. & Bernards, R. Transforming growth factor-β requires its target plasminogen activator inhibitor-1 for cytostatic activity. J. Biol. Chem. 283, 24308–24313 (2008).
Vijayachandra, K., Lee, J. & Glick, A. B. Smad3 regulates senescence and malignant conversion in a mouse multistage skin carcinogenesis model. Cancer Res. 63, 3447–3452 (2003).
Glick, A. B. et al. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 8, 2429–2440 (1994).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Frippiat, C. et al. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-β1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J. Biol. Chem. 276, 2531–2537 (2001).
Debacq-Chainiaux, F. et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-β1 signaling pathway. J. Cell Sci. 118, 743–758 (2005).
Ye, X. et al. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol. Cell 27, 183–196 (2007). Describes the link between Wnt signalling, SAHF and cellular senescence.
Narita, M. et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 126, 503–514 (2006).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Zhang, R. et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005).
Ye, X. et al. Definition of pRB- and p53-dependent and -independent steps in HIRA/ASF1a-mediated formation of senescence-associated heterochromatin foci. Mol. Cell. Biol. 27, 2452–2465 (2007).
Liu, S. et al. Homozygous deletion of glycogen synthase kinase 3β bypasses senescence allowing Ras transformation of primary murine fibroblasts. Proc. Natl Acad. Sci. USA 105, 5248–5253 (2008).
Damalas, A., Kahan, S., Shtutman, M., Ben-Ze'ev, A. & Oren, M. Deregulated β-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 20, 4912–4922 (2001).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Klaus, A. & Birchmeier, W. Wnt signalling and its impact on development and cancer. Nature Rev. Cancer 8, 387–398 (2008).
Fridman, A. L. et al. Expression profiling identifies three pathways altered in cellular immortalization: interferon, cell cycle, and cytoskeleton. J. Gerontol. A Biol. Sci. Med. Sci. 61, 879–889 (2006).
Perera, R. et al. Defining the transcriptome of accelerated and replicatively senescent keratinocytes reveals links to differentiation, interferon signaling, and Notch related pathways. J. Cell. Biochem. 98, 394–408 (2006).
Kulaeva, O. I. et al. Epigenetic silencing of multiple interferon pathway genes after cellular immortalization. Oncogene 22, 4118–4127 (2003).
Tanaka, N. et al. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell 77, 829–839 (1994).
Li, Q. et al. Interferon regulatory factors IRF5 and IRF7 inhibit growth and induce senescence in immortal Li-Fraumeni fibroblasts. Mol. Cancer Res. 6, 770–784 (2008).
Xin, H., Pereira-Smith, O. M. & Choubey, D. Role of IFI 16 in cellular senescence of human fibroblasts. Oncogene 23, 6209–6217 (2004).
Sasaki, M., Ikeda, H., Sato, Y. & Nakanuma, Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic. Res. 42, 625–632 (2008).
Pammer, J. et al. Interferon-α prevents apoptosis of endothelial cells after short-term exposure but induces replicative senescence after continuous stimulation. Lab. Invest. 86, 997–1007 (2006).
Moiseeva, O., Mallette, F. A., Mukhopadhyay, U. K., Moores, A. & Ferbeyre, G. DNA damage signaling and p53-dependent senescence after prolonged β-interferon stimulation. Mol. Biol. Cell 17, 1583–1592 (2006).
Akiyama, M. et al. Interferon-α repressed telomerase along with G1-accumulation of Daudi cells. Cancer Lett. 142, 23–30 (1999).
Kim, T. K. et al. Interferon regulatory factor 3 activates p53-dependent cell growth inhibition. Cancer Lett. 242, 215–221 (2006).
Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, e301 (2008).
Maier, J. A., Voulalas, P., Roeder, D. & Maciag, T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science 249, 1570–1574 (1990).
Hsu, J. Y., Hsu, M. Y., Sorger, T., Herlyn, M. & Levine, E. M. Heparin/endothelial cell growth supplement regulates matrix gene expression and prolongs life span of vascular smooth muscle cells through modulation of interleukin-1. In Vitro Cell Dev. Biol. Anim. 35, 647–654 (1999).
Ancrile, B., Lim, K. H. & Counter, C. M. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 21, 1714–1719 (2007).
Hong, D. S., Angelo, L. S. & Kurzrock, R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer 110, 1911–1928 (2007).
Lu, C., Vickers, M. F. & Kerbel, R. S. Interleukin 6: a fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Proc. Natl Acad. Sci. USA 89, 9215–9219 (1992).
Lu, C. et al. Endogenous interleukin 6 can function as an in vivo growth-stimulatory factor for advanced-stage human melanoma cells. Clin. Cancer Res. 2, 1417–1425 (1996).
Sparmann, A. & Bar-Sagi, D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6, 447–458 (2004). References 78 and 82 demonstrate the pro-tumorigenic contribution of interleukins.
Kunz, C., Pebler, S., Otte, J. & von der Ahe, D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 23, 3710–3717 (1995).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Wakefield, L. M., Smith, D. M., Flanders, K. C. & Sporn, M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J. Biol. Chem. 263, 7646–7654 (1988).
Miyazono, K., Hellman, U., Wernstedt, C. & Heldin, C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J. Biol. Chem. 263, 6407–6415 (1988).
Sato, Y. & Rifkin, D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J. Cell Biol. 109, 309–315 (1989).
MacDonald, R. G. et al. A single receptor binds both insulin-like growth factor II and mannose-6-phosphate. Science 239, 1134–1137 (1988).
Godár, S. et al. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur. J. Immunol. 29, 1004–1013 (1999).
Nykjaer, A. et al. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J. Cell Biol. 141, 815–828 (1998).
Purchio, A. F. et al. Identification of mannose 6-phosphate in two asparagine-linked sugar chains of recombinant transforming growth factor-beta 1 precursor. J. Biol. Chem. 263, 14211–14215 (1988).
Kovacina, K. S. et al. Interactions of recombinant and platelet transforming growth factor-β1 precursor with the insulin-like growth factor II/mannose 6-phosphate receptor. Biochem. Biophys. Res. Comm. 160, 393–403 (1989).
Dennis, P. A. & Rifkin, D. B. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc. Natl Acad. Sci. USA 88, 580–584 (1991).
Leksa, V. et al. TGF-β-induced apoptosis in endothelial cells mediated by M6P/IGFII-R and mini-plasminogen. J. Cell Sci. 118, 4577–4586 (2005).
Annes, J. P., Munger, J. S. & Rifkin, D. B. Making sense of latent TGFβ activation. J. Cell Sci. 116, 217–224 (2003).
Kishimoto, T., Akira, S. & Taga, T. Interleukin-6 and its receptor: a paradigm for cytokines. Science 258, 593–597 (1992).
Duplomb, L. et al. Soluble mannose 6-phosphate/insulin-like growth factor II (IGF-II) receptor inhibits interleukin-6-type cytokine-dependent proliferation by neutralization of IGF-II. Endocrinology 144, 5381–5389 (2003).
Nolan, C. M., Kyle, J. W., Watanabe, H. & Sly, W. S. Binding of insulin-like growth factor II (IGF-II) by human cation-independent mannose 6-phosphate receptor/IGF-II receptor expressed in receptor-deficient mouse L cells. Cell Regul. 1, 197–213 (1990).
Massagué, J., Kelly, B. & Mottola, C. Stimulation by insulin-like growth factors is required for cellular transformation by type beta transforming growth factor. J. Biol. Chem. 260, 4551–4554 (1985).
De Souza, A. T., Hankins, G. R., Washington, M. K., Orton, T. C. & Jirtle, R. L. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nature Genet. 11, 447–449 (1995).
Huang, S. S. et al. Cellular growth inhibition by IGFBP-3 and TGF-β1 requires LRP-1. FASEB J. 17, 2068–2081 (2003).
Leal, S. M., Liu, Q., Huang, S. S. & Huang, J. S. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. J. Biol. Chem. 272, 20572–20576 (1997).
Leal, S. M., Huang, S. S. & Huang, J. S. Interactions of high affinity insulin-like growth factor-binding proteins with the type V transforming growth factor-β receptor in mink lung epithelial cells. J. Biol. Chem. 274, 6711–6717 (1999).
Huang, S. S., Leal, S. M., Chen, C. L., Liu, I. H. & Huang, J. S. Identification of insulin receptor substrate proteins as key molecules for the TβR-V/LRP-1-mediated growth inhibitory signaling cascade in epithelial and myeloid cells. FASEB J. 18, 1719–1721 (2004).
Huang, S. S., Leal, S. M., Chen, C. L., Liu, I. H. & Huang, J. S. Cellular growth inhibition by TGF-beta1 involves IRS proteins. FEBS Lett. 565, 117–121 (2004).
Lalou, C., Silve, C., Rosato, R., Segovia, B. & Binoux, M. Interactions between insulin-like growth factor-I (IGF-I) and the system of plasminogen activators and their inhibitors in the control of IGF-binding protein-3 production and proteolysis in human osteosarcoma cells. Endocrinology 135, 2318–2326 (1994).
Campbell, P. G. & Andress, D. L. Plasmin degradation of insulin-like growth factor-binding protein-5 (IGFBP-5): regulation by IGFBP-5-(201–218). Am. J. Physiol. 273, E996–E1004 (1997).
Nam, T. J., Busby, W. & Clemmons, D. R. Insulin-like growth factor binding protein-5 binds to plasminogen activator inhibitor-I. Endocrinology 138, 2972–2978 (1997).
Herz, J., Clouthier, D. E. & Hammer, R. E. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71, 411–421 (1992).
Olson, D. et al. Internalization of the urokinase-plasminogen activator inhibitor type-1 complex is mediated by the urokinase receptor. J. Biol. Chem. 267, 9129–9133 (1992).
Nykjaer, A. et al. Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J. Biol. Chem. 267, 14543–14546 (1992).
Orth, K., Madison, E. L., Gething, M. J., Sambrook, J. F. & Herz, J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Proc. Natl Acad. Sci. USA 89, 7422–7426 (1992).
Conese, M. et al. α-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J. Cell Biol. 131, 1609–1622 (1995).
Nykjaer, A. et al. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 16, 2610–2620 (1997).
Gonias, S., Wu, L. & Salicioni, A. Low density lipoprotein receptor-related protein: regulation of the plasma membrane proteome. Thromb. Haemost. 91, 1056–1064 (2004).
Fanayan, S., Firth, S. M., Butt, A. J. & Baxter, R. C. Growth inhibition by insulin-like growth factor-binding protein-3 in T47D breast cancer cells requires transforming growth factor-β (TGF-β) and the type II TGF-β receptor. J. Biol. Chem. 275, 39146–39151 (2000).
Fanayan, S., Firth, S. M. & Baxter, R. C. Signaling through the Smad pathway by insulin-like growth factor-binding protein-3 in breast cancer cells. Relationship to transforming growth factor-β 1 signaling. J. Biol. Chem. 277, 7255–7261 (2002).
Gucev, Z. S., Oh, Y., Kelley, K. M. & Rosenfeld, R. G. Insulin-like growth factor binding protein 3 mediates retinoic acid- and transforming growth factor β2-induced growth inhibition in human breast cancer cells. Cancer Res. 56, 1545–1550 (1996).
Gilardoni, M. B. et al. Decreased expression of the low-density lipoprotein receptor-related protein-1 (LRP-1) in rats with prostate cancer. J. Histochem. Cytochem. 51, 1575–1580 (2003).
Gagnon, A. M., Chabot, J., Pardasani, D. & Sorisky, A. Extracellular matrix induced by TGFβ impairs insulin signal transduction in 3T3-L1 preadipose cells. J. Cell Physiol. 175, 370–378 (1998).
Mincione, G. et al. TGF-β 1 modulation of IGF-I signaling pathway in rat thyroid epithelial cells. Exp. Cell Res. 287, 411–423 (2003).
Weigert, C. et al. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J. Biol. Chem. 281, 7060–7067 (2006).
Senn, J. J., Klover, P. J., Nowak, I. A. & Mooney, R. A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51, 3391–3399 (2002).
Rotter, V., Nagaev, I. & Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278, 45777–45784 (2003).
Andreozzi, F. et al. Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol. Cell. Biol. 27, 2372–2383 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Lewis, A. M., Varghese, S., Xu, H. & Alexander, H. R. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J. Transl. Med. 4, 48 (2006).
Yuan, A., Chen, J. J., Yao, P. L. & Yang, P. C. The role of interleukin-8 in cancer cells and microenvironment interaction. Front. Biosci. 10, 853–865 (2005).
Yang, G. et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA 103, 16472–16477 (2006). This paper shows that a secreted cytokine can induce senescence in the stroma, thereby promoting tumorigenesis.
Krtolica, A. & Campisi, J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int. J. Biochem. Cell Biol. 34, 1401–1414 (2002).
Herbig, U., Ferreira, M., Condel, L., Carey, D. & Sedivy, J. M. Cellular senescence in aging primates. Science 311, 1257 (2006).
Adachi, Y., Yoshio-Hoshino, N. & Nishimoto, N. The blockade of IL-6 signaling in rational drug design. Curr. Pharm. Des. 14, 1217–1224 (2008).
Ancrile, B. B., O'Hayer, K. M. & Counter, C. M. Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Mol. Interv. 8, 22–27 (2008).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001). This article shows that factors secreted by senescent cells can contribute to tumorigenesis.
Paradis, V. et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum. Pathol. 32, 327–332 (2001).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Kirkwood, T. B. & Austad, S. N. Why do we age? Nature 408, 233–238 (2000).
Cosme-Blanco, W. et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 8, 497–503 (2007).
Feldser, D. M. & Greider, C. W. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 11, 461–469 (2007).
Matheu, A. et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 (2007).
Himi, T., Yoshioka, I. & Kataura, A. Influence of age on the production of interleukin-8-like chemokine (GRO/CINC-1) in rat nasal mucosa. Eur. Arch. Otorhinolaryngol. 254, 101–104 (1997).
Carrieri, G. et al. The G/C915 polymorphism of transforming growth factor β1 is associated with human longevity: a study in Italian centenarians. Aging Cell 3, 443–448 (2004).
Breese, C. R., Ingram, R. L. & Sonntag, W. E. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J. Gerontol. 46, B180–B187 (1991).
Acknowledgements
We are grateful to R. van Doorn, R. Kortlever, P. ten Dijke and T. Geiger for helpful suggestions and critical reading of the manuscript, and J. Campisi for kindly sharing data before publication. T.K. and D.S.P. are supported by grants from the Dutch Cancer Society (KWF) including a Queen Wilhelmina Program grant, and the Netherlands Organization for Scientific Research (NWO). D.S.P. is also supported by the EMBO Young Investigator Program.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Kringle domains
-
Protein domains that fold into large loops stabilized by three disulphide linkages and are important for interaction of proteins with blood coagulation factors.
- Antagonistic pleiotropy
-
The hypothesis that genes with beneficial effects early in life are favoured by selection even if they have detrimental effects at later ages.
Rights and permissions
About this article
Cite this article
Kuilman, T., Peeper, D. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9, 81–94 (2009). https://doi.org/10.1038/nrc2560
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2560
This article is cited by
-
Cancer-associated fibroblasts in radiotherapy: Bystanders or protagonists?
Cell Communication and Signaling (2023)
-
The senescence difference between the central and peripheral cornea induced by sutures
BMC Ophthalmology (2023)
-
Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging
Nature Aging (2023)
-
Sexual dimorphic metabolic and cognitive responses of C57BL/6 mice to Fisetin or Dasatinib and quercetin cocktail oral treatment
GeroScience (2023)
-
NF-κB, a culprit of both inflamm-ageing and declining immunity?
Immunity & Ageing (2022)