Abstract
The increasing number of cancer survivors is cause for celebration, but this expanding population has highlighted the problem of tumour dormancy, which can lead to relapse. As we start to understand more about the biology of dormant cancer cells, we can begin to address how best to treat this form of disease. Preclinical models and initial clinical trials, as exemplified in patients with breast cancer, are paving the way to address how best to treat long-term cancer survivors to minimize the risk of cancer recurrence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
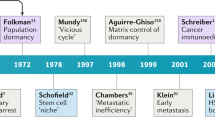
Weiss, L. Metastatic inefficiency. Adv. Cancer Res. 54, 159–211 (1990).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002).
Heyn, C. et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med. 56, 1001–1010 (2006).
Townson, J. L. et al. Three-dimensional imaging and quantification of both solitary cells and metastases in whole mouse liver by magnetic resonance imaging. Cancer Res. 69, 8326–8331 (2009).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Naumov, G. N. et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 62, 2162–2168 (2002).
Cameron, M. D. et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60, 2541–2546 (2000).
Holmgren, L., O'Reilly, M. S. & Folkman, J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Med. 1, 149–153 (1995).
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nature Rev. Cancer 7, 834–846 (2007).
Muller, V., Alix-Panabieres, C. & Pantel, K. Insights into minimal residual disease in cancer patients: implications for anti-cancer therapies. Eur. J. Cancer 46, 1189–1197 (2010).
Pantel, K., Alix-Panabieres, C. & Riethdorf, S. Cancer micrometastases. Nature Rev. Clin. Oncol. 6, 339–351 (2009).
Naumov, G. N. et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J. Natl Cancer Inst. 98, 316–325 (2006).
Naumov, G. N., Akslen, L. A. & Folkman, J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5, 1779–1787 (2006).
Indraccolo, S., Favaro, E. & Amadori, A. Dormant tumors awaken by a short-term angiogenic burst: the spike hypothesis. Cell Cycle 5, 1751–1755 (2006).
Favaro, E., Amadori, A. & Indraccolo, S. Cellular interactions in the vascular niche: implications in the regulation of tumor dormancy. APMIS 116, 648–659 (2008).
Indraccolo, S. et al. Cross-talk between tumor and endothelial cells involving the Notch3-Dll4 interaction marks escape from tumor dormancy. Cancer Res. 69, 1314–1323 (2009).
Ranganathan, A. C., Adam, A. P., Zhang, L. & Aguirre-Ghiso, J. A. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol. Ther. 5, 729–735 (2006).
Allgayer, H. & Aguirre-Ghiso, J. A. The urokinase receptor (u-PAR)-a link between tumor cell dormancy and minimal residual disease in bone marrow? APMIS 116, 602–614 (2008).
Adam, A. P. et al. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 69, 5664–5672 (2009).
Rak, J. W., McEachern, D. & Miller, F. R. Sequential alteration of peanut agglutinin binding-glycoprotein expression during progression of murine mammary neoplasia. Br. J. Cancer 65, 641–648 (1992).
Morris, V. L., Tuck, A. B., Wilson, S. M., Percy, D. & Chambers, A. F. Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin. Exp. Metastasis 11, 103–112 (1993).
Naumov, G. N. et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res. Treat. 82, 199–206 (2003).
Barkan, D. et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008).
Barkan, D. et al. Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716 (2010).
Barkan, D., Green, J. E. & Chambers, A. F. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 46, 1181–1188 (2010).
Shibue, T. & Weinberg, R. A. Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl Acad. Sci. USA 106, 10290–10295 (2009).
Goodison, S. et al. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin. Cancer Res. 9, 3808–3814 (2003).
Suzuki, M., Mose, E. S., Montel, V. & Tarin, D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am. J. Pathol. 169, 673–681 (2006).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010).
Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Sargent, D. J. et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J. Clin. Oncol. 25, 4569–4574 (2007).
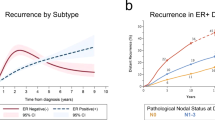
Saphner, T., Tormey, D. C. & Gray, R. Annual hazard rates of recurrence for breast cancer after primary therapy. J. Clin. Oncol. 14, 2738–2746 (1996).
Retsky, M. W., Demicheli, R., Hrushesky, W. J., Baum, M. & Gukas, I. D. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 116, 730–741 (2008).
Hanin, L. & Korosteleva, O. Does extirpation of the primary breast tumor give boost to growth of metastases? Evidence revealed by mathematical modeling. Math. Biosci. 223, 133–141 (2010).
Riethmuller, G. & Klein, C. A. Early cancer cell dissemination and late metastatic relapse: clinical reflections and biological approaches to the dormancy problem in patients. Semin. Cancer Biol. 11, 307–311 (2001).
Demicheli, R., Terenziani, M. & Bonadonna, G. Estimate of tumor growth time for breast cancer local recurrences: rapid growth after wake-up? Breast Cancer Res. Treat. 51, 133–137 (1998).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Ragaz, J. et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N. Engl. J. Med. 337, 956–962 (1997).
Overgaard, M. et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N. Engl. J. Med. 337, 949–955 (1997).
Slade, M. J. et al. Comparison of bone marrow, disseminated tumour cells and blood-circulating tumour cells in breast cancer patients after primary treatment. Br. J. Cancer 100, 160–166 (2009).
Wikman, H., Vessella, R. & Pantel, K. Cancer micrometastasis and tumour dormancy. APMIS 116, 754–770 (2008).
Flores, L. M. et al. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br. J. Cancer 102, 1495–1502 (2010).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nature Rev. Cancer 4, 448–456 (2004).
Kendal, W. S. Chance mechanisms affecting the burden of metastases. BMC Cancer 5, 138 (2005).
Chambers, A. F. Influence of diet on metastasis and tumor dormancy. Clin. Exp. Metastasis 26, 61–66 (2009).
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005).
Fisher, B. et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J. Natl Cancer Inst. 88, 1529–1542 (1996).
Fisher, B., Dignam, J., Bryant, J. & Wolmark, N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J. Natl Cancer Inst. 93, 684–690 (2001).
Stewart, H. J. et al. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. The Scottish Cancer Trials Breast Group. Br. J. Cancer 74, 297–299 (1996).
Tormey, D. C., Gray, R. & Falkson, H. C. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J. Natl Cancer Inst. 88, 1828–1833 (1996).
Delozier, T. et al. Delayed adjuvant tamoxifen: ten-year results of a collaborative randomized controlled trial in early breast cancer (TAM-02 trial). Ann. Oncol. 11, 515–519 (2000).
Goss, P. E. et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 349, 1793–1802 (2003).
Goss, P. E. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl Cancer Inst. 97, 1262–1271 (2005).
Goss, P. E. et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J. Clin. Oncol. 26, 1948–1955 (2008).
Sabnis, G., Goloubeva, O., Gilani, R., Macedo, L. & Brodie, A. Sensitivity to the aromatase inhibitor letrozole is prolonged after a “break” in treatment. Mol. Cancer Ther. 9, 46–56 (2010).
Moy, B. & Goss, P. E. TEACH: Tykerb evaluation after chemotherapy. Clin. Breast Cancer 7, 489–492 (2007).
Tsao, H., Cosimi, A. B. & Sober, A. J. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer 79, 2361–2370 (1997).
Farrar, J. D. et al. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-γ in establishing and maintaining the tumor-dormant state. J. Immunol. 162, 2842–2849 (1999).
Muller-Hermelink, N. et al. TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13, 507–518 (2008).
Quesnel, B. Tumor dormancy and immunoescape. APMIS 116, 685–694 (2008).
Zhu, D., Corral, L. G., Fleming, Y. W. & Stein, B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol. Immunother. 57, 1849–1859 (2008).
Delea, T. E. et al. Cost-effectiveness of extended adjuvant letrozole therapy after 5 years of adjuvant tamoxifen therapy in postmenopausal women with early-stage breast cancer. Am. J. Manag. Care 12, 374–386 (2006).
Chapman, J. A. et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J. Natl Cancer Inst. 100, 252–260 (2008).
Whelan, T. J. et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J. Clin. Oncol. 23, 6931–6940 (2005).
Pantel, K., Braun, S., Schlimok, G. & Riethmuller, G. Micrometastatic tumour cells in bone marrow in colorectal cancer. Lancet 341, 501 (1993).
Pantel, K. et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl Cancer Inst. 85, 1419–1424 (1993).
Murphy, J. E. & Ryan, D. P. American Society of Clinical Oncology 2010 colorectal update. Expert Rev. Anticancer Ther. 10, 1371–1373 (2010).
Allegra, C. J. et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J. Clin. Oncol. 27, 3385–3390 (2009).
Wolmark, N. et al. A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: results of NSABP Protocol C-08. J. Clin. Oncol. Abstr. 27, LBA4 (2009).
Acknowledgements
P.E.G. is supported by the Avon Foundation for Women in New York, USA. A.F.C. is Canada Research Chair in Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Goss, P., Chambers, A. Does tumour dormancy offer a therapeutic target?. Nat Rev Cancer 10, 871–877 (2010). https://doi.org/10.1038/nrc2933
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2933
This article is cited by
-
A cell cycle centric view of tumour dormancy
British Journal of Cancer (2023)
-
KISS1 metastasis suppressor in tumor dormancy: a potential therapeutic target for metastatic cancers?
Cancer and Metastasis Reviews (2023)
-
Cancer stem cells: an insight into the development of metastatic tumors and therapy resistance
Stem Cell Reviews and Reports (2023)
-
In vivo metabolic imaging identifies lipid vulnerability in a preclinical model of Her2+/Neu breast cancer residual disease and recurrence
npj Breast Cancer (2022)
-
Repeat Resection for Advanced Colorectal Liver Metastases—Does it have the Potential for Cure?
World Journal of Surgery (2022)