Key Points
-
Chemotherapeutic alkylating agents induce a range of cytotoxic and mutagenic adducts onto DNA.
-
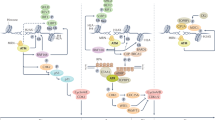
Alkylating agent-induced damage to DNA is sensed and repaired by different cellular mechanisms, including direct repair by the AlkB homologue (ALKBH) family and O6-methylguanine-DNA methyltransferase (MGMT) proteins or by pathways such as base excision repair (BER), mismatch repair (MMR), homologous recombination, Fanconi anaemia and translesion DNA synthesis (TLS).
-
Diverse cellular pathways collectively modulate alkylation sensitivity, and imbalances within or between these pathways can have deleterious consequences at the cellular and whole-animal levels.
-
Developing agents to modulate DNA repair pathways is a promising strategy to increase the effectiveness of current chemotherapeutic alkylating agents.
-
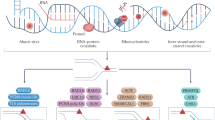
Investigation of DNA repair capacity in cancer patients may provide crucial information about which chemotherapeutic therapies to use, because differences can result in drastic variation in alkylation sensitivity.
Abstract
Alkylating agents constitute a major class of frontline chemotherapeutic drugs that inflict cytotoxic DNA damage as their main mode of action, in addition to collateral mutagenic damage. Numerous cellular pathways, including direct DNA damage reversal, base excision repair (BER) and mismatch repair (MMR), respond to alkylation damage to defend against alkylation-induced cell death or mutation. However, maintaining a proper balance of activity both within and between these pathways is crucial for a favourable response of an organism to alkylating agents. Furthermore, the response of an individual to alkylating agents can vary considerably from tissue to tissue and from person to person, pointing to genetic and epigenetic mechanisms that modulate alkylating agent toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ballschmiter, K. Pattern and sources of naturally produced organohalogens in the marine environment: biogenic formation of organohalogens. Chemosphere 52, 313–324 (2003).
Hecht, S. S. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. 424, 127–142 (1999).
Hamilton, J. T., McRoberts, W. C., Keppler, F., Kalin, R. M. & Harper, D. B. Chloride methylation by plant pectin: an efficient environmentally significant process. Science 301, 206–209 (2003).
Rydberg, B. & Lindahl, T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1, 211–216 (1982).
Taverna, P. & Sedgwick, B. Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J. Bacteriol. 178, 5105–5111 (1996).
Bartsch, H., O'Neill, I. K., Schulte-Hermann, R. & International Agency for Research on Cancer. Relevance of N-Nitroso Compounds to Human Cancer: Exposures and Mechanisms (Oxford Univ. Press distributor, Lyon, London, 1987).
Kufe, D. W. et al. (eds) Cancer Medicine, 6th edn (BC Decker, Hamilton, Canada, 2003).
Shrivastav, N., Li, D. & Essigmann, J. M. Chemical biology of mutagenesis and DNA repair: cellular responses to DNA alkylation. Carcinogenesis 31, 59–70 (2010).
Drablos, F. et al. Alkylation damage in DNA and RNA-repair mechanisms and medical significance. DNA Repair 3, 1389–1407 (2004).
Johnson, R. E., Yu, S.-L., Prakash, S. & Prakash, L. A Role for yeast and human translesion synthesis DNA polymerases in promoting replication through 3-methyl adenine. Mol. Cell. Biol. 27, 7198–7205 (2007).
Engelward, B. P. et al. A chemical and genetic approach together define the biological consequences of 3-methyladenine lesions in the mammalian genome. J. Biol. Chem. 273, 5412–5418 (1998).
Gate, L. & Tew, K. D. in Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures (ed. Minev, B. R.) 61–85 (Springer Netherlands, 2011).
Sedgwick, B., Bates, P. A., Paik, J., Jacobs, S. C. & Lindahl, T. Repair of alkylated DNA: recent advances. DNA Repair 6, 429–442 (2007).
Samson, L., Thomale, J. & Rajewsky, M. F. Alternative pathways for the in vivo repair of O6-alkylguanine and O4-alkylthymine in Escherichia coli: the adaptive response and nucleotide excision repair. EMBO J. 7, 2261–2267 (1988).
Huang, J. C., Hsu, D. S., Kazantsev, A. & Sancar, A. Substrate spectrum of human excinuclease: repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc. Natl Acad. Sci. USA 91, 12213–12217 (1994).
Hanawalt, P. Subpathways of nucleotide excision repair and their regulation. Oncogene 21, 8949–8956 (2002).
Kaina, B., Christmann, M., Naumann, S. & Roos, W. P. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 6, 1079–1099 (2007).
Deans, A. J. & West, S. C. DNA interstrand crosslink repair and cancer. Nature Rev. Cancer 11, 467–480 (2011).
Kee, Y. & D'Andrea, A. D. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 24, 1680–1694 (2010).
Lange, S. S., Takata, K. & Wood, R. D. DNA polymerases and cancer. Nature Rev. Cancer 11, 96–110 (2011). References 16–20 describe additional pathways that are important in the repair and tolerance of DNA alkylation damage that were not described in detail in this Review.
Svilar, D., Goellner, E. M., Almeida, K. H. & Sobol, R. W. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid. Redox Signal. 14, 2491–2507 (2011).
Monti, P. et al. Rev1 and Polzeta influence toxicity and mutagenicity of Me-lex, a sequence selective N3-adenine methylating agent. DNA Repair 7, 431–438 (2008).
Monti, P. et al. Mutagenicity of N3-methyladenine: a multi-translesion polymerase affair. Mutat. Res. 683, 50–56 (2010).
Roos, W. P. et al. The translesion polymerase Rev3L in the tolerance of alkylating anticancer drugs. Mol. Pharmacol. 76, 927–934 (2009). This paper and references 10, 22 and 23 show that TLS polymerases have a crucial role in determining alkylating agent sensitivity through the bypass of replication-blocking 3meA lesions.
Prasad, R., Shock, D. D., Beard, W. A. & Wilson, S. H. Substrate channeling in mammalian base excision repair pathways: passing the baton. J. Biol. Chem. 285, 40479–40488 (2010).
Mol, C. D., Izumi, T., Mitra, S. & Tainer, J. A. DNA-bound structures and mutants reveal abasic DNA binding by APE1 DNA repair and coordination. Nature 403, 451–456 (2000). The structural and mutational analysis described in this paper provided the basis of the mechanism that toxic BER intermediates are passed from one BER enzyme to another, as reviewed in reference 25.
Sieber, O. M. et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N. Engl. J. Med. 348, 791–799 (2003).
Fleischmann, C. et al. Comprehensive analysis of the contribution of germline MYH variation to early-onset colorectal cancer. Int. J. Cancer 109, 554–558 (2004).
Rusyn, I. et al. Transcriptional networks in S. cerevisiae linked to an accumulation of base excision repair intermediates. PLoS ONE 2, e1252 (2007).
Meira, L. B. et al. Aag-initiated base excision repair drives alkylation-induced retinal degeneration in mice. Proc. Natl Acad. Sci. USA 106, 888–893 (2009). This paper was the first to illustrate that deficiency of a DNA glycosylase in vivo results in protection from alkylation damage, specifically MMS-mediated retinal degeneration.
Sobol, R. W. et al. Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J. Biol. Chem. 278, 39951–39959 (2003).
Fishel, M. L., He, Y., Smith, M. L. & Kelley, M. R. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin. Cancer Res. 13, 260–267 (2007).
Kaasen, I., Evensen, G. & Seeberg, E. Amplified expression of the tag+ and alkA+ genes in Escherichia coli: identification of gene products and effects on alkylation resistance. J. Bacteriol. 168, 642–647 (1986).
Xiao, W. & Samson, L. In vivo evidence for endogenous DNA alkylation damage as a source of spontaneous mutation in eukaryotic cells. Proc. Natl Acad. Sci. USA 90, 2117–2121 (1993).
Glassner, B. J., Rasmussen, L. J., Najarian, M. T., Posnick, L. M. & Samson, L. D. Generation of a strong mutator phenotype in yeast by imbalanced base excision repair. Proc. Natl Acad. Sci. USA 95, 9997–10002 (1998). References 33–35 illustrate that BER intermediates are toxic and mutagenic; an imbalance in the BER pathway results in increased mutagenesis, as well as in enhanced alkylation sensitivity.
Posnick, L. M. & Samson, L. D. Imbalanced base excision repair increases spontaneous mutation and alkylation sensitivity in Escherichia coli. J. Bacteriol. 181, 6763–6771 (1999).
Calleja, F., Jansen, J. G., Vrieling, H., Laval, F. & van Zeeland, A. A. Modulation of the toxic and mutagenic effects induced by methyl methanesulfonate in Chinese hamster ovary cells by overexpression of the rat N-alkylpurine-DNA glycosylase. Mutat. Res. 425, 185–194 (1999).
Rinne, M., Caldwell, D. & Kelley, M. R. Transient adenoviral N-methylpurine DNA glycosylase overexpression imparts chemotherapeutic sensitivity to human breast cancer cells. Mol. Cancer Ther. 3, 955–967 (2004).
Rinne, M. L., He, Y., Pachkowski, B. F., Nakamura, J. & Kelley, M. R. N-methylpurine DNA glycosylase overexpression increases alkylation sensitivity by rapidly removing non-toxic 7-methylguanine adducts. Nucleic Acids Res. 33, 2859–2867 (2005).
Trivedi, R. N. et al. Human methyl purine DNA glycosylase and DNA polymerase β expression collectively predict sensitivity to temozolomide. Mol. Pharmacol. 74, 505–516 (2008).
Coquerelle, T., Dosch, J. & Kaina, B. Overexpression of N-methylpurine-DNA glycosylase in Chinese hamster ovary cells renders them more sensitive to the production of chromosomal aberrations by methylating agents-a case of imbalanced DNA repair. Mutat. Res. 336, 9–17 (1995).
Klapacz, J. et al. Frameshift mutagenesis and microsatellite instability induced by human alkyladenine DNA glycosylase. Mol. Cell 37, 843–853 (2010).
Hofseth, L. J. et al. The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation. J. Clin. Invest. 112, 1887–1894 (2003). This paper clearly describes a correlation between imbalanced BER and microsatellite instability in human tissues.
Cerda, S. R., Turk, P. W., Thor, A. D. & Weitzman, S. A. Altered expression of the DNA repair protein, N-methylpurine-DNA glycosylase (MPG) in breast cancer. FEBS Lett. 431, 12–18 (1998).
Curtis, C. D., Thorngren, D. L. & Nardulli, A. M. Immunohistochemical analysis of oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues. BMC Cancer 10, 9 (2010).
Kim, N. K. et al. Expression of the DNA repair enzyme, N-methylpurine-DNA glycosylase (MPG) in astrocytic tumors. Anticancer Res. 23, 1417–1423 (2003).
Mirabello, L. et al. A comprehensive candidate gene approach identifies genetic variation associated with osteosarcoma. BMC Cancer 11, 209 (2011).
Elder, R. H. et al. Alkylpurine-DNA-N-glycosylase knockout mice show increased susceptibility to induction of mutations by methyl methanesulfonate. Mol. Cell. Biol. 18, 5828–5837 (1998).
Engelward, B. P. et al. Base excision repair deficient mice lacking the Aag alkyladenine DNA glycosylase. Proc. Natl Acad. Sci. USA 94, 13087–13092 (1997).
Roth, R. B. & Samson, L. D. 3-Methyladenine DNA glycosylase-deficient Aag null mice display unexpected bone marrow alkylation resistance. Cancer Res. 62, 656–660 (2002). This paper reports the surprising discovery that AAG deficiency can protect against MMS toxicity in ex vivo bone marrow assays.
Cardinal, J. W. et al. Increased susceptibility to streptozotocin-induced β-cell apoptosis and delayed autoimmune diabetes in alkylpurine-DNA-N-glycosylase-deficient mice. Mol. Cell. Biol. 21, 5605–5613 (2001).
Burns, N. & Gold, B. The effect of 3-methyladenine DNA glycosylase-mediated DNA repair on the induction of toxicity and diabetes by the β-cell toxicant streptozotocin. Toxicol. Sci. 95, 391–400 (2007).
Paik, J., Duncan, T., Lindahl, T. & Sedgwick, B. Sensitization of human carcinoma cells to alkylating agents by small interfering RNA suppression of 3-alkyladenine-DNA glycosylase. Cancer Res. 65, 10472–10477 (2005).
Engelward, B. P. et al. Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO J. 15, 945–952 (1996). References 49 and 54 describe the generation of Aag−/− embryonic stem cells and Aag−/− mice, and show that in certain cell types AAG deficiency results in increased alkylation sensitivity.
Wirtz, S. et al. Both base excision repair and O6-methylguanine-DNA methyltransferase protect against methylation-induced colon carcinogenesis. Carcinogenesis 31, 2111–2117 (2010).
Meira, L. B. et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 118, 2516–2525 (2008).
Liu, L., Taverna, P., Whitacre, C. M., Chatterjee, S. & Gerson, S. L. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin. Cancer Res. 5, 2908–2917 (1999).
Loeb, L. A. & Preston, B. D. Mutagenesis by Apurinic/Apyrimidinic Sites. Annu. Rev. Genet. 20, 201–230 (1986).
Kunz, B. A. et al. Specificity of the mutator caused by deletion of the yeast structural gene (APN1) for the major apurinic endonuclease. Proc. Natl Acad. Sci. USA 91, 8165–8169 (1994).
Luo, M. & Kelley, M. R. Inhibition of the human apurinic/apyrimidinic endonuclease (APE1) repair activity and sensitization of breast cancer cells to DNA alkylating agents with lucanthone. Anticancer Res. 24, 2127–2134 (2004).
Wang, D., Luo, M. & Kelley, M. R. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol. Cancer Ther. 3, 679–686 (2004).
Taverna, P. et al. Methoxyamine potentiates DNA single strand breaks and double strand breaks induced by temozolomide in colon cancer cells. Mutat. Res. 485, 269–281 (2001).
Wilson, D. & Simeonov, A. Small molecule inhibitors of DNA repair nuclease activities of APE1. Cell. Mol. Life Sci. 67, 3621–3631 (2010).
Sobol, R. W. et al. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 379, 183–186 (1996).
Cabelof, D. C. et al. Base excision repair deficiency caused by polymerase β haploinsufficiency: accelerated DNA damage and increased mutational response to carcinogens. Cancer Res. 63, 5799–5807 (2003).
Sobol, R. W. et al. Mutations associated with base excision repair deficiency and methylation-induced genotoxic stress. Proc. Natl Acad. Sci. USA 99, 6860–6865 (2002).
Poltoratsky, V., Horton, J. K., Prasad, R. & Wilson, S. H. REV1 mediated mutagenesis in base excision repair deficient mouse fibroblast. DNA Repair 4, 1182–1188 (2005).
Horton, J. K., Joyce-Gray, D. F., Pachkowski, B. F., Swenberg, J. A. & Wilson, S. H. Hypersensitivity of DNA polymerase β null mouse fibroblasts reflects accumulation of cytotoxic repair intermediates from site-specific alkyl DNA lesions. DNA Repair 2, 27–48 (2003).
Sobol, R. W. et al. The lyase activity of the DNA repair protein β-polymerase protects from DNA-damage-induced cytotoxicity. Nature 405, 807–810 (2000). Reference 64 illustrates how Pol β is required for BER and that Pol β deficiency results in hypersensitivity to alkylating agents. Mutational analysis described in reference 69 demonstrates that the 5′-dRP lyase activity of Pol β is sufficient to protect against alkylating agent toxicity.
Wang, L., Bhattacharyya, N., Rabi, T. & Banerjee, S. Mammary carcinogenesis in transgenic mice expressing a dominant-negative mutant of DNA polymerase β in their mammary glands. Carcinogenesis 28, 1356–1363 (2007).
Chan, K. et al. Overexpression of DNA polymerase β results in an increased rate of frameshift mutations during base excision repair. Mutagenesis 22, 183–188 (2007).
Yamada, N. A. & Farber, R. A. Induction of a low level of microsatellite instability by overexpression of DNA polymerase β. Cancer Res. 62, 6061–6064 (2002).
Almeida, K. H. & Sobol, R. W. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair 6, 695–711 (2007).
Ström, C. E. et al. Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res. 39, 3166–3175 (2010).
Zdzienicka, M. Z. et al. A Chinese hamster ovary cell mutant (EM-C11) with sensitivity to simple alkylating agents and a very high level of sister chromatid exchanges. Mutagenesis 7, 265–269 (1999).
Horton, J. K. et al. XRCC1 and DNA polymerase β in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 18, 48–63 (2008).
Briegert, M. & Kaina, B. Human monocytes, but not dendritic cells derived from them, are defective in base excision repair and hypersensitive to methylating agents. Cancer Res. 67, 26–31 (2007).
Jiang, Y., Zhou, S., Sandusky, G. E., Kelley, M. R. & Fishel, M. L. Reduced expression of DNA repair and redox signaling protein APE1/Ref-1 impairs human pancreatic cancer cell survival, proliferation, and cell cycle progression. Cancer Invest. 28, 885–895 (2010).
Al-Attar, A. et al. Human apurinic/apyrimidinic endonuclease (APE1) is a prognostic factor in ovarian, gastro-oesophageal and pancreatico-biliary cancers. Br. J. Cancer 102, 704–709 (2010).
Di Maso, V. et al. Subcellular localization of APE1/Ref-1 in human hepatocellular carcinoma: possible prognostic significance. Mol. Med. 13, 89–96 (2007).
Freitas, S., Moore, D. H., Michael, H. & Kelley, M. R. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer. Clin. Cancer Res. 9, 4689–4694 (2003).
Kakolyris, S. et al. Nuclear localization of human AP endonuclease 1 (HAP1/Ref-1) associates with prognosis in early operable non-small cell lung cancer (NSCLC). The J. Pathol. 189, 351–357 (1999).
Sweasy, J. B., Lang, T. & DiMaio, D. Is base excision repair a tumor suppressor mechanism? Cell Cycle 5, 250–259 (2006).
Starcevic, D., Dalal, S. & Sweasy, J. B. Is there a link between DNA polymerase β and cancer? Cell Cycle 3, 996–999 (2004).
Jiang, J., Zhang, X., Yang, H. & Wang, W. Polymorphisms of DNA repair genes: ADPRT, XRCC1, and XPD and cancer risk in genetic epidemiology. Methods Mol. Biol. 471, 305–333 (2009).
Simeonov, A. et al. Identification and characterization of inhibitors of human apurinic/apyrimidinic endonuclease APE1. PLoS ONE 4, e5740 (2009).
Bapat, A. et al. Novel small-molecule inhibitor of apurinic/apyrimidinic endonuclease 1 blocks proliferation and reduces viability of glioblastoma cells. J. Pharmacol. Exp. Ther. 334, 988–998 (2010).
Tang, J.-b. et al. N-methylpurine DNA glycosylase and DNA polymerase β modulate BER inhibitor potentiation of glioma cells to temozolomide. Neuro Oncol. 13, 471–486 (2011).
Liu, L. & Gerson, S. L. Therapeutic impact of methoxyamine: blocking repair of abasic sites in the base excision repair pathway. Curr. Opin. Investig. Drugs 5, 623–627 (2004).
Jiang, Y. et al. Role of APE1 in differentiated neuroblastoma SH-SY5Y cells in response to oxidative stress: use of APE1 small molecule inhibitors to delineate APE1 functions. DNA Repair 8, 1273–1282 (2009).
Izumi, T. et al. Two essential but distinct functions of the mammalian abasic endonuclease. Proc. Natl Acad. Sci. USA 102, 5739–5743 (2005). This study clearly showed that APE contains two domains with independent functions, both of which are essential for cell survival.
Wilson, S. et al. Base excision repair and design of small molecule inhibitors of human DNA polymerase β. Cell. Mol. Life Sci. 67, 3633–3647 (2010). References 89 and 92 illustrate the strategies and progress made in developing drugs that inhibit BER as cancer chemotherapeutic agents.
Hu, H. Y. et al. Identification of small molecule synthetic inhibitors of DNA polymerase β by NMR chemical shift mapping. J. Biol. Chem. 279, 39736–39744 (2004).
Jaiswal, A. S. et al. A novel inhibitor of DNA polymerase β enhances the ability of temozolomide to impair the growth of colon cancer cells. Mol. Cancer Res. 7, 1973–1983 (2009).
Jaiswal, A. S. et al. DNA polymerase β as a novel target for chemotherapeutic intervention of colorectal cancer. PLoS ONE 6, e16691 (2011).
Stachelek, G. C. et al. Potentiation of temozolomide cytotoxicity by inhibition of DNA polymerase β is accentuated by BRCA2 mutation. Cancer Res. 70, 409–417 (2010).
Rouleau, M., Patel, A., Hendzel, M. J., Kaufmann, S. H. & Poirier, G. G. PARP inhibition: PARP1 and beyond. Nature Rev. Cancer 10, 293–301 (2010).
Mégnin-Chanet, F., Bollet, M. & Hall, J. Targeting poly(ADP-ribose) polymerase activity for cancer therapy. Cell. Mol. Life Sci. 67, 3649–3662 (2010).
de Murcia, J. M. et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl Acad. Sci. USA 94, 7303–7307 (1997).
Shibata, A. et al. Parp-1 deficiency causes an increase of deletion mutations and insertions/rearrangements in vivo after treatment with an alkylating agent. Oncogene 24, 1328–1337 (2005).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005). References 101 and 102 are seminal papers that describe synthetic lethality following treatment of BRCA-deficient cells with PARP inhibitors, which has since resulted in clinical trials testing the efficacy of PARP inhibitors in patients with breast cancer, as reviewed in reference 97.
Jelezcova, E. et al. Parp1 activation in mouse embryonic fibroblasts promotes Pol β-dependent cellular hypersensitivity to alkylation damage. Mutat. Res. 686, 57–67 (2010).
Kelley, M. R. DNA repair inhibitors: where do we go from here? DNA Repair 10, 1183–1185 (2011).
Pegg, A. E. Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools. Chem. Res. Toxicol. 24, 618–639 (2011).
Nakatsuru, Y. et al. O6-methylguanine-DNA methyltransferase protects against nitrosamine-induced hepatocarcinogenesis. Proc. Natl Acad. Sci. USA 90, 6468–6472 (1993).
Dumenco, L. L., Allay, E., Norton, K. & Gerson, S. L. The prevention of thymic lymphomas in transgenic mice by human O6-alkylguanine-DNA alkyltransferase. Science 259, 219–222 (1993). References 106 and 107 were the first to demonstrate that transgenic overexpression of MGMT in mice confers resistance to alkylating agent-induced carcinogenesis and reduced tumour formation.
Liu, L., Allay, E., Dumenco, L. L. & Gerson, S. L. Rapid repair of O6-methylguanine-DNA adducts protects transgenic mice from N-methylnitrosourea-induced thymic lymphomas. Cancer Res. 54, 4648–4652 (1994).
Allay, E., Veigl, M. & Gerson, S. L. Mice over-expressing human O6 alkylguanine-DNA alkyltransferase selectively reduce O6 methylguanine mediated carcinogenic mutations to threshold levels after N-methyl-N-nitrosourea. Oncogene 18, 3783–3787 (1999).
Zhou, Z. Q. et al. Spontaneous hepatocellular carcinoma is reduced in transgenic mice overexpressing human O6- methylguanine-DNA methyltransferase. Proc. Natl Acad. Sci. USA 98, 12566–12571 (2001).
Zaidi, N. H. et al. Transgenic expression of human MGMT protects against azoxymethane-induced aberrant crypt foci and G to A mutations in the K-ras oncogene of mouse colon. Carcinogenesis 16, 451–456 (1995).
Becker, K., Dosch, J., Gregel, C. M., Martin, B. A. & Kaina, B. Targeted expression of human O6-methylguanine-DNA methyltransferase (MGMT) in transgenic mice protects against tumor initiation in two-stage skin carcinogenesis. Cancer Res. 56, 3244–3249 (1996).
Becker, K. et al. DNA repair protein MGMT protects against N-methyl-N-nitrosourea-induced conversion of benign into malignant tumors. Carcinogenesis 24, 541–546 (2003).
Becker, K., Gregel, C. M. & Kaina, B. The DNA repair protein O6-methylguanine-DNA methyltransferase protects against skin tumor formation induced by antineoplastic chloroethylnitrosourea. Cancer Res. 57, 3335–3338 (1997).
Allay, E. et al. Potentiation of lymphomagenesis by methylnitrosourea in mice transgenic for LMO1 is blocked by O6-alkylguanine DNA-alkyltransferase. Oncogene 15, 2127–2132 (1997).
Qin, X., Zhou, H., Liu, L. & Gerson, S. L. Transgenic expression of human MGMT blocks the hypersensitivity of PMS2-deficient mice to low dose MNU thymic lymphomagenesis. Carcinogenesis 20, 1667–1673 (1999).
Qin, X., Liu, L. & Gerson, S. L. Mice defective in the DNA mismatch gene PMS2 are hypersensitive to MNU induced thymic lymphoma and are partially protected by transgenic expression of human MGMT. Oncogene 18, 4394–4400 (1999).
Reese, J. S., Allay, E. & Gerson, S. L. Overexpression of human O6-alkylguanine DNA alkyltransferase (AGT) prevents MNU induced lymphomas in heterozygous p53 deficient mice. Oncogene 20, 5258–5263 (2001).
Kisby, G. E. et al. DNA repair modulates the vulnerability of the developing brain to alkylating agents. DNA Repair 8, 400–412 (2009).
Tsuzuki, T. et al. Targeted disruption of the DNA repair methyltransferase gene renders mice hypersensitive to alkylating agent. Carcinogenesis 17, 1215–1220 (1996).
Glassner, B. J. et al. DNA repair methyltransferase (Mgmt) knockout mice are sensitive to the lethal effects of chemotherapeutic alkylating agents. Mutagenesis 14, 339–347 (1999).
Klapacz, J. et al. O6-methylguanine-induced cell death involves exonuclease 1 as well as DNA mismatch recognition in vivo. Proc. Natl Acad. Sci. USA 106, 576–581 (2009).
Reese, J. S., Qin, X., Ballas, C. B., Sekiguchi, M. & Gerson, S. L. MGMT expression in murine bone marrow is a major determinant of animal survival after alkylating agent exposure. J. Hematother. Stem Cell Res. 10, 115–123 (2001).
Sakumi, K. et al. Methylnitrosourea-induced tumorigenesis in MGMT gene knockout mice. Cancer Res. 57, 2415–2418 (1997).
Iwakuma, T. et al. High incidence of nitrosamine-induced tumorigenesis in mice lacking DNA repair methyltransferase. Carcinogenesis 18, 1631–1635 (1997).
Hansen, R. J., Nagasubramanian, R., Delaney, S. M., Samson, L. D. & Dolan, M. E. Role of O6-methylguanine-DNA methyltransferase in protecting from alkylating agent-induced toxicity and mutations in mice. Carcinogenesis 28, 1111–1116 (2007).
Christmann, M., Verbeek, B., Roos, W. P. & Kaina, B. O6-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochim. Biophys. Acta 1816, 179–190 (2011). This comprehensive review summarizes the vast number of studies on MGMT expression, activity and promoter methylation in human tissues and cancers.
Hongeng, S. et al. O6-Methylguanine-DNA methyltransferase protein levels in pediatric brain tumors. Clin. Cancer Res. 3, 2459–2463 (1997).
Bobola, M. S. et al. O6-methylguanine-DNA methyltransferase in pediatric primary brain tumors. Clin. Cancer Res. 7, 613–619 (2001).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
Christmann, M. et al. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int. J. Cancer 127, 2106–2118 (2010).
Kaina, B., Margison, G. & Christmann, M. Targeting O6-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy. Cell. Mol. Life Sci. 67, 3663–3681 (2010).
Weingart, J. et al. Phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-benzylguanine in adults with recurrent malignant glioma: new approaches to brain tumor therapy CNS consortium trial. J. Clin. Oncol. 25, 399–404 (2007).
Quinn, J. A. et al. Phase II trial of temozolomide plus O6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J. Clin. Oncol. 27, 1262–1267 (2009).
Batts, E. et al. O6-benzylguanine and BCNU in multiple myeloma: a phase II trial. Cancer Chemother. Pharmacol. 60, 415–421 (2007).
Gajewski, T. F. et al. Phase II trial of the O6-alkylguanine DNA alkyltransferase inhibitor O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea in advanced melanoma. Clin. Cancer Res. 11, 7861–7865 (2005).
Moritz, T., Mackay, W., Glassner, B. J., Williams, D. A. & Samson, L. Retrovirus-mediated expression of a DNA repair protein in bone marrow protects hematopoietic cells from nitrosourea-induced toxicity in vitro and in vivo. Cancer Res. 55, 2608–2614 (1995).
Maze, R. et al. Increasing DNA repair methyltransferase levels via bone marrow stem cell transduction rescues mice from the toxic effects of 1,3-bis(2-chloroethyl)-1-nitrosourea, a chemotherapeutic alkylating agent. Proc. Natl Acad. Sci. USA 93, 206–210 (1996). References 137 and 138 show that transplantation of bone marrow cells overexpressing MGMT can suppress alkylating agent myelosuppression and toxicity in mice.
Jelinek, J. et al. Long-term protection of hematopoiesis against the cytotoxic effects of multiple doses of nitrosourea by retrovirus-mediated expression of human O6-alkylguanine-DNA-alkyltransferase. Blood 87, 1957–1961 (1996).
Allay, J. A., Koç, O. N., Davis, B. M. & Gerson, S. L. Retroviral-mediated gene transduction of human alkyltransferase complementary DNA confers nitrosourea resistance to human hematopoietic progenitors. Clin. Cancer Res. 2, 1353–1359 (1996).
Schambach, A. & Baum, C. Vector design for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. DNA Repair 6, 1187–1196 (2007).
Neff, T. et al. Methylguanine methyltransferase–mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model. J. Clin. Invest. 112, 1581–1588 (2003).
Maze, R., Hanenberg, H. & Williams, D. A. Establishing chemoresistance in hematopoietic progenitor cells. Mol. Med. Today 3, 350–358 (1997).
Ragg, S., Xu-Welliver, M. & Bailey, J. Direct reversal of DNA damage by mutant methyltransferase protein protects mice against dose-intensified chemotherapy and leads to in vivo selection of hematopoietic stem cells. Cancer Res. 60, 5187–5195 (2000).
Jansen, M. et al. Hematoprotection and enrichment of transduced cells in vivo after gene transfer of MGMTP140K into hematopoietic stem cells. Cancer Gene Ther. 9, 737–746 (2002).
Neff, T. et al. Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood 105, 997–1002 (2005).
Reha-Krantz, L. J., Nonay, R. L., Day, R. S. & Wilson, S. H. Replication of O6-methylguanine-containing DNA by repair and replicative DNA polymerases. J. Biol. Chem. 271, 20088–20095 (1996).
Haracska, L., Prakash, S. & Prakash, L. Replication past O6-methylguanine by yeast and human DNA polymerase eta. Mol. Cell. Biol. 20, 8001–8007 (2000).
Choi, J. Y. et al. Translesion synthesis across O6-alkylguanine DNA adducts by recombinant human DNA polymerases. J. Biol. Chem. 281, 38244–38256 (2006).
Takenaka, K. et al. Involvement of vertebrate Polκ in translesion DNA synthesis across DNA monoalkylation damage. J. Biol. Chem. 281, 2000–2004 (2006).
Haracska, L., Prakash, S. & Prakash, L. Yeast DNA polymerase ζ is an efficient extender of primer ends opposite from 7,8-dihydro-8-oxoguanine and O6-methylguanine. Mol. Cell. Biol. 23, 1453–1459 (2003).
Haracska, L., Prakash, L. & Prakash, S. Role of human DNA polymerase κ as an extender in translesion synthesis. Proc. Natl Acad. Sci. USA 99, 16000–16005 (2002).
Kaina, B., Gerhard, F., Sankar, M. & Coquerelle, T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis 12, 1857–1867 (1991).
Lips, J. & Kaina, B. Repair of O6-methylguanine is not affected by thymine base pairing and the presence of MMR proteins. DNA Repair 487, 59–66 (2001).
Casorelli I, Russo MT & M., B. Role of mismatch repair and MGMT in response to anticancer therapies. Anticancer Agents Med. Chem. 8, 368–380 (2008).
Duckett, D. R. et al. Human MutSα recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc. Natl Acad. Sci. USA 93, 6443–6447 (1996).
Meikrantz, W., Bergom, M. A., Memisoglu, A. & Samson, L. O6-alkylguanine DNA lesions trigger apoptosis. Carcinogenesis 19, 369–372 (1998).
Rasmussen, L. J. & Samson, L. The Escherichia coli MutS DNA mismatch binding protein specifically binds O6-methylguanine DNA lesions. Carcinogenesis 17, 2085–2088 (1996). References 156 and 158 provide a direct link between the MMR pathway and alkylation damage by showing that MUTS and MUTSα can directly recognize O6meG lesions.
Dosch, J., Christmann, M. & Kaina, B. Mismatch G-T binding activity and MSH2 expression is quantitatively related to sensitivity of cells to methylating agents. Carcinogenesis 19, 567–573 (1998).
Hickman, M. J. & Samson, L. D. Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc. Natl Acad. Sci. USA 96, 10764–10769 (1999).
Mojas, N., Lopes, M. & Jiricny, J. Mismatch repair-dependent processing of methylation damage gives rise to persistent single-stranded gaps in newly replicated DNA. Genes Dev. 21, 3342–3355 (2007).
Ochs, K. & Kaina, B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. 60, 5815–5824 (2000). References 161 and 162 provide evidence that MMR-dependent processing of O6meG gives rise to cytotoxic DSBs that trigger apoptotic cell death.
Rajesh, P., Rajesh, C., Wyatt, M. D. & Pittman, D. L. RAD51D protects against MLH1-dependent cytotoxic responses to O6-methylguanine. DNA Repair 9, 458–467 (2010).
Roos, W. P. et al. Brca2/Xrcc2 dependent HR, but not NHEJ, is required for protection against O6-methylguanine triggered apoptosis, DSBs and chromosomal aberrations by a process leading to SCEs. DNA Repair 8, 72–86 (2009).
Cejka, P. & Jiricny, J. Interplay of DNA repair pathways controls methylation damage toxicity in saccharomyces cerevisiae. Genetics 179, 1835–1844 (2008).
Mirzoeva, O. K., Kawaguchi, T. & Pieper, R. O. The Mre11/Rad50/Nbs1 complex interacts with the mismatch repair system and contributes to temozolomide-induced G2 arrest and cytotoxicity. Mol. Cancer Ther. 5, 2757–2766 (2006).
Tsaryk, R., Fabian, K., Thacker, J. & Kaina, B. Xrcc2 deficiency sensitizes cells to apoptosis by MNNG and the alkylating anticancer drugs temozolomide, fotemustine and mafosfamide. Cancer Lett. 239, 305–313 (2006).
Eich, M., Roos, W. P., Dianov, G. L., Digweed, M. & Kaina, B. Nijmegen breakage syndrome protein (NBN) causes resistance to methylating anticancer drugs such as temozolomide. Mol. Pharmacol. 78, 943–951 (2010).
Kaina, B. The interrelationship between SCE induction, cell survival, mutagenesis, aberration formation and DNA synthesis inhibition in V79 cells treated with N-methyl-N-nitrosourea or N-methyl-N'-nitro-N-nitrosoguanidine. Mutat. Res. 142, 49–54 (1985).
Yoshioka, K.-i., Yoshioka, Y. & Hsieh, P. ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-methylguanine adducts. Mol. Cell 22, 501–510 (2006).
Branch, P., Aquilina, G., Bignami, M. & Karran, P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature 362, 652–654 (1993).
Koi, M. et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 54, 4308–4312 (1994).
de Wind, N., Dekker, M., Berns, A., Radman, M. & te Riele, H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82, 321–330 (1995). References 171–173 show that loss of MMR confers resistance to alkylating agent toxicity in both cells and animals, providing conclusive evidence for the hypothesis that inappropriate MMR-dependent processing of O6meG is a major cytotoxic event associated with alkylating agents.
Andrew, S. E. et al. Tissues of MSH2-deficient mice demonstrate hypermutability on exposure to a DNA methylating agent. Proc. Natl Acad. Sci. USA 95, 1126–1130 (1998).
Hickman, M. J. & Samson, L. D. Apoptotic signaling in response to a single type of DNA lesion, O6-methylguanine. Mol. Cell 14, 105–116 (2004).
Toft, N. J. et al. Msh2 status modulates both apoptosis and mutation frequency in the murine small intestine. Proc. Natl Acad. Sci. USA 96, 3911–3915 (1999).
Sansom, O. J., Toft, N. J., Winton, D. J. & Clarke, A. R. Msh-2 suppresses in vivo mutation in a gene dose and lesion dependent manner. Oncogene 20, 3580–3584 (2001).
Sansom, O. J. & Clarke, A. R. The ability to engage enterocyte apoptosis does not predict long-term crypt survival in p53 and Msh2 deficient mice. Oncogene 21, 5934–5939 (2002).
Reese, J. S., Liu, L. & Gerson, S. L. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood 102, 1626–1633 (2003).
Sansom, O. J. et al. Apoptosis and mutation in the murine small intestine: loss of Mlh1- and Pms2-dependent apoptosis leads to increased mutation in vivo. DNA Repair 2, 1029–1039 (2003).
Bugni, J. M., Meira, L. B. & Samson, L. D. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene 28, 734–741 (2009).
Kawate, H. et al. A defect in a single allele of the Mlh1 gene causes dissociation of the killing and tumorigenic actions of an alkylating carcinogen in methyltransferase-deficient mice. Carcinogenesis 21, 301–305 (2000).
Stallons, L. J. & McGregor, W. G. Translesion synthesis polymerases in the prevention and promotion of carcinogenesis. J. Nucleic Acids 2010, 1–10 (2010).
Moynahan, M. E. & Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nature Rev. Mol. Cell Biol. 11, 196–207 (2010).
Arana, M. E. & Kunkel, T. A. Mutator phenotypes due to DNA replication infidelity. Semin. Cancer Biol. 20, 304–311 (2010).
Hewish, M., Lord, C. J., Martin, S. A., Cunningham, D. & Ashworth, A. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nature Rev. Clin. Oncol. 7, 197–208 (2010).
Trainer, A. H. et al. The role of BRCA mutation testing in determining breast cancer therapy. Nature Rev. Clin. Oncol. 7, 708–717 (2010).
Valeri, N. et al. Modulation of mismatch repair and genomic stability by miR-155. Proc. Natl Acad. Sci. USA 107, 6982–6987 (2010).
Moskwa, P. et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol. Cell 41, 210–220 (2011). This study shows that microRNAs involved in the expression of DNA damage proteins could be modulated in combination with PARP inhibitors to affect sensitivity to alkylating agents.
Kiltie, A. E. (ed.) Molecular Epidemiology of DNA Repair Genes in Bladder Cancer (Humana Press, Bethesda, 2009).
Fenske, T. S. et al. Identification of candidate alkylator-induced cancer susceptibility genes by whole genome scanning in mice. Cancer Res. 66, 5029–5038 (2006).
Lutz, W. A true threshold dose in chemical carcinogenesis cannot be defined for a population, irrespective of the mode of action. Hum. Exp. Toxicol. 19, 566–568 (2000).
Fry, R. C. et al. Genomic predictors of interindividual differences in response to DNA damaging agents. Genes Dev. 22, 2621–2626 (2008).
Begley, T. J., Rosenbach, A. S., Ideker, T. & Samson, L. D. Hot spots for modulating toxicity identified by genomic phenotyping and localization mapping. Mol. Cell 16, 117–125 (2004).
Ravi, D. et al. A network of conserved damage survival pathways revealed by a genomic RNAi screen. PLoS Genet. 5, e1000527 (2009).
McKinnon, P. J. DNA repair deficiency and neurological disease. Nature Rev. Neurosci. 10, 100–112 (2009).
Robbins JH, Kraemer KH, Lutzner MA, Festoff BW & HG., C. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann. Intern. Med. 80, 221–248 (1974).
Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749–1753 (1995).
Lovell, M. A., Xie, C. & Markesbery, W. R. Decreased base excision repair and increased helicase activity in Alzheimer's disease brain. Brain Res. 855, 116–123 (2000).
Weissman, L., de Souza-Pinto, N. C., Mattson, M. P. & Bohr, V. A. DNA base excision repair activities in mouse models of Alzheimer's disease. Neurobiol. Aging 30, 2080–2081 (2009).
Iida, T., Furuta, A., Nishioka, K., Nakabeppu, Y. & Iwaki, T. Expression of 8-oxoguanine DNA glycosylase is reduced and associated with neurofibrillary tangles in Alzheimer's disease brain. Acta Neuropathol. 103, 20–25 (2002).
Weissman, L. et al. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 35, 5545–5555 (2007).
Hegde, M. L., Hazra, T. K. & Mitra, S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 18, 27–47 (2008).
Zecca, L., Youdim, M. B. H., Riederer, P., Connor, J. R. & Crichton, R. R. Iron, brain ageing and neurodegenerative disorders. Nature Rev. Neurosci. 5, 863–873 (2004).
Interthal, H. et al. SCAN1 mutant Tdp1 accumulates the enzyme-DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 24, 2224–2233 (2005).
El-Khamisy, S. F. et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434, 108–113 (2005). This important paper was the first to link SSB repair with neurodegeneration.
Lee, Y. et al. The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Nature Neurosci. 12, 973–980 (2009).
Lauritzen, K. H. et al. Mitochondrial DNA toxicity in forebrain neurons causes apoptosis, neurodegeneration, and impaired behavior. Mol. Cell. Biol. 30, 1357–1367 (2010).
Liu, L., Qin, X. & Gerson, S.L. Reduced lung tumorigenesis in human methylguanine DNA — methyltransferase transgenic mice achieved by expression of transgene within target cell. Carcinogenesis 20, 279–284 (1999).
Acknowledgements
The authors would like to thank members of the Samson Laboratory and C.-L. Fu for critical reading of this manuscript. The authors have been supported by US National Institutes of Health grants CA055042, CA075576, CA149261, CA112967, DP1-OD006422 and ES002109. D.F. was supported by an American Cancer Society Postdoctoral Fellowship and L.D.S. is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Alkyl
-
Chemical sidechain that consists only of single-bonded carbon and hydrogen atoms; for example, a methyl or an ethyl group.
- Nucleophilic substitution
-
Chemical bonding reaction between an electron pair donor (nucleophile) with an electron pair acceptor (electrophile).
- Chloroethyl
-
Alkyl functional group consisting of a chlorine atom bonded to an ethyl carbon group.
- Depurination
-
Loss of a purine base (adenine or guanine) from the DNA backbone through chemical or enzymatic hydrolysis.
- Clastogenic
-
The ability to disrupt or to break chromosomes.
- Sister chromatid exchanges
-
(SCEs). Crossing-over event between sister chromatids, leading to the exchange of homologous stretches of DNA sequence.
- Microsatellite instability
-
Mutations in short motifs of tandemly repeated nucleotides that result from replication slippage and deficient mismatch repair.
- Pancytopenia
-
Severe reduction in the number of all blood cell types, including red and white blood cells and platelets.
- Myelosuppression
-
Inhibition of blood cell production in the bone marrow.
- Apoptosis
-
A type of caspase-dependent programmed cell death that is characterized by cell blebbing and DNA fragmentation.
Rights and permissions
About this article
Cite this article
Fu, D., Calvo, J. & Samson, L. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer 12, 104–120 (2012). https://doi.org/10.1038/nrc3185
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3185
This article is cited by
-
Base flipping mechanism and binding strength of methyl-damaged DNA during the interaction with AGT
Journal of Biological Physics (2024)
-
A deep dive into transmissible cancer evolution in bivalve mollusks
Nature Cancer (2023)
-
Regulation of base excision repair during adipogenesis and osteogenesis of bone marrow-derived mesenchymal stem cells
Scientific Reports (2023)
-
Structural and mechanistic insights into the DNA glycosylase AAG-mediated base excision in nucleosome
Cell Discovery (2023)
-
Duplex sequencing provides detailed characterization of mutation frequencies and spectra in the bone marrow of MutaMouse males exposed to procarbazine hydrochloride
Archives of Toxicology (2023)