Key Points
-
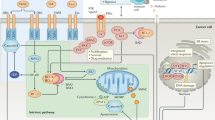
Tumour-necrosis factor (TNF) was discovered many years ago as a serum factor that was able to kill cancer cells in mice. The TNF receptor (TNFR) was shown to be expressed by mammalian cells years later, and led to the discovery of a superfamily of transmembrane proteins. These discoveries led to the identification of two gene families that include 18 ligands and 28 receptors, many of which are being targeted as anticancer therapies.
-
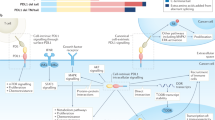
TNFR signalling was discovered to be an important aspect of the immune response, and family members such as FASL and APO2L/TRAIL induce apoptosis through a p53-independent mechanism. The signalling members of the TNFR superfamily can be divided into two main subgroups on the basis of their cytoplasmic region. One class of receptors, called death receptors (DR), contains a cytoplasmic death domain, whereas the other class does not.
-
APO2L/TRAIL has been shown to induce apoptosis in a wide variety of cancer cells, whereas most normal human cell types are resistant to APO2L/TRAIL-induced cell death.
-
Some TNFR family members do not signal, but act as 'decoys' that compete with receptors for ligands. A number of tumour types overexpress decoy receptors.
-
Treatment with factors that activate death-receptor signalling on cancer cells, and antibodies or small molecules that antagonize decoy receptors, might be an effective anticancer strategy.
Abstract
Cancer cells often develop resistance to chemotherapy or irradiation through mutations in the p53 tumour-suppressor gene, which prevent apoptosis induction in response to cellular damage. Death receptors — members of the tumour-necrosis factor receptor (TNFR) superfamily — signal apoptosis independently of p53. Decoy receptors, by contrast, are a non-signalling subset of the TNFR superfamily that attenuate death-receptor function. Agents that are designed to activate death receptors (or block decoy receptors) might therefore be used to kill tumour cells that are resistant to conventional cancer therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
O'Malley, W. E., Achinstein, B. & Shear, M. J. Action of bacterial polysaccharide on tumors II: damage of sarcoma 37 by serum of mice treated with Serratia marscescens polysaccharide and induced tolerance. J. Natl Cancer Inst. 29, 1169–1175 (1962).
Carswell, E. A. et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA 72, 3666–3670 (1975).
Helson, L., Green, S., Carswell, E. & Old, L. J. Effect of tumor necrosis factor on cultured melanoma cells. Nature 258, 731–732 (1975).
Pennica, D., Nedwin, G. E., Hayflick, J., Aggarwal, B. B. & Goeddel, D. V. Human tumor necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature 312, 724–729 (1984).
Gray, P. W. et al. Cloning and expression of cDNA for human lymphotoxin, a lymphkine with tumor necrosis activity. Nature 312, 721–724 (1984).
Beutler, B. et al. Identity of tumor necrosis factor and the macrophage-secreted factor cachectin. Nature 316, 552–554 (1985).
Browning, J. L. et al. Lymphotoxin-β, a novel member of the TNF family that forms a heterotrimeric complex with lymphotoxin on the cell surface. Cell 72, 847–856 (1993).
Aggarwal, B. B., Eessalu, T. E. & Hass, P. E. Characterization of receptors for tumor necrosis factor and their regulation by γ-interferon. Nature 318, 665–667 (1985).
Stamenkovic, I., Clark, E. A. & Seed, B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 8, 1403–1410 (1989).
Kwon, B. S. & Weissman, S. M. cDNA sequences of two inducible T-cell genes. Proc. Natl Acad. Sci. USA 86, 1963–1967 (1989).
Johnson, D. et al. Expression and structure of the human NGF receptor. Cell 47, 545–554 (1986).
Tartaglia, L. & Goeddel, D. Two TNF receptors. Immunol. Today 13, 151–153 (1992).
Smith, C. A., Farrah, T. & Goodwin, R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76, 959–962 (1994).
Wiley, S. R. et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3, 673–682 (1995).
Pitti, R. M. et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor receptor family. J. Biol. Chem. 271, 12697–12690 (1996).References 14 and 15 provided the first identification and characterization of APO2L/TRAIL as an apoptosis-inducing ligand.
Ashkenazi, A. & Dixit, V. M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Wallach, D. Cytokine Reference:TNF Ligand and TNF/NGF Receptor Families (Academic, San Diego, 2000).
Yan, M. et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr. Biol. 11, 1547–1552 (2001).
Thompson, J. S. et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293, 2108–2111 (2001).
Wiley, S. R. et al. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 15, 837–846 (2001).
Locksley, R. M., Killeen, N. & Lenardo, M. J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Eck, M. J. & Sprang, S. R. The structure of tumor necrosis factor-α at 2.6Å resolution. J. Biol. Chem. 264, 17595–17605 (1989).
Eck, M. J., Ultsch, M., Rinderknecht, E., de Vos, A. M. & Sprang, S. R. The structure of human lymphotoxin (tumor-necrosis factor-β) at 1. 9Å resolution. J. Biol. Chem. 267, 2119–2122 (1992).
Hymowitz, S. G. et al. A unique zinc-binding site revealed by a high-resolution X-ray structure of homotrimeric Apo2L/TRAIL. Biochemistry 39, 633–640 (2000).
Karpusas, M. et al. 2Å crystal structure of an extracellular fragment of human CD40 ligand. Structure 3, 1031–1039 (1995).
Lam, J., Nelson, C. A., Ross, F. P., Teitelbaum, S. L. & Fremont, D. H. Crystal structure of the TRANCE/RANKL cytokine reveals determinants of receptor-ligand specificity. J. Clin. Invest. 108, 971–979 (2001).
Idriss, H. T. & Naismith, J. H. TNF and the TNF receptor superfamily: structure-function relationship(s). Microsc. Res. Tech. 50, 184–195 (2000).
Chinnaiyan, A. M., O'Rourke, K., Tewari, M. & Dixit, V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505–512 (1995).
Boldin, M. P. et al. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270, 387–391 (1995).
Hsu, H., Xiong, J. & Goeddel, D. The TNF receptor-associated protein TRADD signals cell death and NF-κB activation. Cell 81, 495–504 (1995).
Adams, J. & Cory, S. The Bcl-2 protein family: arbiters of cell survival. Science 281, 1322–1326 (1998).
Green, D. Apoptotic pathways: paper wraps stone blunts scissors. Cell 102, 1–4 (2000).
Hunt, A. & Evan, G. Till death us do part. Science 293, 1784–1785 (2001).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to an antagonizing IAP protein. Cell 102, 43–53 (2000).
Wallach, D. et al. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17, 331–367 (1999).
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).
Emery, J. G. et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 273, 14363–14367 (1998).
Pitti, R. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396, 699–703 (1998).Reports the first identification of DcR3 and shows its interaction with FASL and its overexpression in tumours.
Yu, K. et al. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J. Biol. Chem. 274, 13733–13736 (1999).
Migone, T.-S. et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16, 479–492 (2002).
Hersh, E. M. et al. Phase II studies of recombinant human tumor necrosis factor-α in patients with malignant disease: a summary of the Southwest Oncology Group experience. J. Immunother. 10, 426–431 (1991).
Eggermont, A. M. M. TNF registered in Europe: does TNF get a second chance? J. Immunother. 23, 505–506 (2000).
Alexander, H. R. J. The effects of limb perfusion with tumor necrosis factor on circulating levels of proinflammatory cytokines. J. Immunother. 24, 285–286 (2001).
Wielockx, B. et al. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nature Med. 7, 1202–1208 (2001).Provides a potential approach to mitigating the systemic toxicity of TNF and improving its therapeutic index.
Stoelcker, B., Hehlgans, T., Bluethmann, H., Luther, T. & Mannel, D. N. Tumor necrosis factor induces tumor necrosis via tumor necrosis factor receptor type-1-expressing endothelial cells of the tumor vasculature. Am. J. Pathol. 156, 1171–1176 (2000).Shows that, in a mouse fibrosarcoma model, TNF exerts its tumour-necrotizing effect indirectly, by acting through TNFR1 on tumour endothelial cells.
Pan, G. et al. The receptor for the cytotoxic ligand TRAIL. Science 276, 111–113 (1997).
Sheridan, J. P. et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277, 818–821 (1997).
Pan, G. et al. An antagonist decoy receptor and a new death domain-containing receptor for TRAIL. Science 277, 815–818 (1997).
Walczak, H. et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 16, 5386–5397 (1997).
Marsters, S. A. et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr. Biol. 7, 1003–1006 (1997).
Degli-Esposti, M. et al. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 186, 1165–1170 (1997).
Degli-Esposti, M. A. et al. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7, 813–820 (1997).
Truneh, A. et al. Temperature-sensitive differential affinity of TRAIL for its receptors. J. Biol. Chem. 275, 23319–23325 (2000).
Cretney, E. et al. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 168, 1356–1361 (2002).
Takeda, K. et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J. Exp. Med. 195, 161–169 (2002).References 55 and 56 show that inhibition of APO2L/TRAIL by gene knockout or neutralizing antibody increases the incidence and metastasis of mutagen-induced tumours in mice, indicating that this ligand is involved in immune surveillance against tumours.
Johnsen, A. C. et al. Regulation of APO2 ligand/trail expression in NK cells — involvement in NK cell-mediated cytotoxicity. Cytokine 11, 664–672 (1999).
Kayagaki, N. et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J. Immunol. 163, 1906–1913 (1999).
Griffith, T. et al. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J. Exp. Med. 189, 1343–1353 (1999).
Kayagaki, N. et al. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J. Immunol. 162, 2639–2647 (1999).
Mariani, S. & Krammer, P. Surface expression of TRAIL/Apo2 ligand in activated mouse T and B cells. Eur. J. Immunol. 28, 1492–1498 (1998).
Jeramias, I., Herr, I., Boehler, T. & Debatin, K. M. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 28, 143–152 (1998).
Thomas, W. D. & Hersey, P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J. Immunol. 161, 2195–2200 (1998).
Martinez-Lorenzo, M. J. et al. Involvement of Apo2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur. J. Immunol. 28, 2714–2725 (1998).
Kayagaki, N. et al. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 189, 1451–1460 (1999).
Chen, Q. et al. Induction of Apo2L and modulation of Bcl-2-related proteins regulate Type I interferon-induced apoptosis in multiple myeloma. Blood 98, 2183–2192 (2001).
Altucci, L. et al. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nature Med. 7, 680–686 (2001).
Gong, B. & Almasan, A. Genomic organization and transcriptional regulation of human Apo2L/TRAIL gene. Biochem. Biophys. Res. Commun. 278, 747–752 (2000).
Clarke, P. et al. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74, 8135–8139 (2000).
Secchiero, P. et al. Human herpesvirus 7 induces the functional upregulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) coupled to TRAIL-R1 down-modulation in CD4+ T cells. Blood 15, 2474–2481 (2001).
Sato, K. et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur. J. Immunol. 31, 3138–3146 (2001).
Monleon, I. et al. Differential secretion of Fas ligand or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J. Immunol. 167, 6736–6744 (2001).
Mariani, S. & Krammer, P. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur. J. Immunol. 28, 973–982 (1998).
Hymowitz, S. G. et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Molec. Cell 4, 563–571 (1999).
Bodmer, J.-L., Meier, P., Tschopp, J. & Schneider, P. Cysteine 230 is essential for the structure and activity of the cytotoxic ligand TRAIL. J. Biol. Chem. 275, 20632–20637 (2000).
Ashkenazi, A. et al. Safety and anti-tumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 104, 155–162 (1999).
Walczak, H. et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Med. 5, 157–163 (1999).References 76 and 77 provided the first evidence that soluble APO2L/TRAIL proteins induce tumour-cell apoptosis in vivo . Reference 76 described the non-tagged, trimeric APO2L/TRAIL variant and showed its efficacy as a single agent and its synergy with chemotherapy against tumour xenografts. Reference 77 described a modified leucine-zipper-fused variant and showed its single-agent activity.
Rieger, J., Naumann, U., Glaser, T., Ashkenazi, A. & Weller, M. Apo2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 427, 124–128 (1998).
Keane, M. M., Ettenberg, S. A., Nau, M. M., Russel, E. & Lipkowitz, S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 59, 734–741 (1999).
Mizutani, Y., Yoshida, O., Miki, T. & Bonavida, B. Synergistic cytotoxicity and apoptosis by Apo-2 ligand and adriamycin against bladder cancer cells. Clin. Cancer Res. 5, 2605–2612 (1999).
Gazitt, Y. TRAIL is a potent inducer of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia 13, 1817–1824 (1999).
Yu, R., Mandlekar, S., Ruben, S., Ni, J. & Kong, A.-N. T. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in androgen-independent prostate cancer cells. Cancer Res. 60, 2384–2389 (2000).
Mitsiades, N., Poulaki, V., Mitsiades, C. & Tsokos, M. Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5xs. Cancer Res. 61, 2704–2712 (2001).
Ashkenazi, A. & Dixit, V. M. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11, 255–260 (1999).
Kelley, S. K. et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 299, 31–38 (2001).
Mitsiades, C. S. et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 98, 795–804 (2001).
Roth, W. et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in a thymic mice in the absence of neurotoxicity. Biochem. Biophys. Res. Commun. 265, 1999 (1999).
Pollack, I. F., Erff, M. & Ashkenazi, A. Direct stimulation of apoptotic signaling by soluble Apo2L/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin. Cancer Res. 7, 1362–1369 (2001).
Gliniak, B. & Le, T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 59, 6153–6158 (1999).
Chinnaiyan, A. M. et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc. Natl Acad. Sci. USA 97, 1754–1759 (2000).
Chuntharapai, A. et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J. Immunol. 166, 4891–4898 (2001).
Ichikawa, K. et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte toxicity. Nature Med. 7, 954–960 (2001).
Trauth, B. C. et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245, 301–304 (1989).
Lawrence, D. et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nature Med. 7, 383–385 (2001).
Jo, M. et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nature Med. 6, 564–567 (2000).
Qin, J. Z., Chaturvedi, V., Bonish, B. & Nickoloff, B. J. Avoiding premature apoptosis of normal epidermal cells. Nature Med. 7, 385–386 (2001).References 94–96 together show that non-tagged, zinc-containing APO2L/TRAIL trimers have little toxicity towards cultured hepatocytes or keratinocytes, whereas tagged, non-optimized versions of the ligand induce apoptosis in these cell types.
Muhlenbeck, F. et al. The tumor necrosis factor-related apoptosis-inducing ligand receptors TRAIL-R1 and TRAIL-R2 have distinct cross-linking requirements for initiation of apoptosis and are non-redundant in JNK activation. J. Biol. Chem. 275, 32208–32213 (2000).
Evan, G. & Littlewood, T. A matter of life and cell death. Science 281, 1317–1322 (1998).
Mongkolsapaya, J. et al. Lymphocyte inhibitor of TRAIL: a new receptor protecting lymphocytes from the death ligand TRAIL. J. Immunol. 160, 3–6 (1998).
Zhang, X. D., Nguyen, T., Thomas, W. D., Sanders, J. E. & Hersey, P. Mechanisms of resistance of normal cells to TRAIL-induced apoptosis vary between different cell types. FEBS Lett. 482, 193–199 (2000).
Bernard, D., Quatannes, B., Vundenbunder, B. & Abbadie, C. Rel/NF-κB transcription factors protect against tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor DcR1. J. Biol. Chem. 276, 27322–27328 (2001).
Kagawa, S. et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 61, 3330–3338 (2001).
Bai, C. et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc. Natl Acad. Sci. USA 97, 1230–1235 (2000).
Oshima, K. et al. Amplification and expression of a decoy receptor for Fas ligand (DcR3) in virus (EBV or HTLV-1) associated lymphomas. Cancer Lett. 160, 89–97 (2000).
Roth, W. et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 61, 2759–2765 (2001).References 103–105 confirm and extend the observation that DcR3 is overexpressed in various tumours and that it acts as a decoy for FASL.
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
French, L. E. & Tschopp, J. Inhibition of death receptor signaling by FLICE-inhibitory protein as a mechanism for immune escape of tumors. J. Exp. Med. 190, 891–893 (1999).
Berke, G. The CTL's kiss of death. Cell Death Differ. 81, 9–12 (1995).
Medema, J., de Jong, J., van Hall, T., Melief, C. & Offringa, R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J. Exp. Med. 190, 1033–1038 (1999).
Djerbi, M. et al. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190, 1025–1031 (1999).References 109 and 110 show that ectopic expression of FLIP, an intracellular inhibitor of death-receptor signalling, enables tumour cells to escape immune surveillance in mouse models. This is summarized in reference 107.
Alderson, M. R. et al. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 178, 2231–2235 (1993).
Tamada, K. et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nature Med. 6, 283–289 (2000).
Roonie, I. A. et al. The lymphotoxin-β receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J. Biol. Chem. 275, 14307–14315 (2000).
Li, H., Zhu, H., Xu, C.-J. & Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway to apoptosis. Cell 94, 491–501 (1998).
Luo, X., Budihardjo, I., Zou, H., Slaughter, C. & Wang, X. Bid, a Bcl2-interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 (1998).
Gross, A. et al. Caspase cleaved Bid targets mitochondria and is required for cytochrome c release, while Bcl-xL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274, 1156–1163 (1999).
Yin, X.-M. et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 (1999).
Eskes, R., Desagher, S., Antonsson, B. & Martinou, J. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20, 929–935 (2000).
Wei, M. et al. tBID, a membrane targeted death ligand, oligomerizes Bak to release cytochrome c. Genes Dev. 14, 2060–2071 (2000).
El-Deiry, W. S. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 8, 1066–1075 (2001).
Scaffidi, C. et al. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274, 22532–22538 (1999).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Zhang, L., Yu, J., Park, B., Kinzler, K. & Vogelstein, B. Role of BAX in the apoptotic response to anticancer agents. Science 290, 989–992 (2000).
LeBlanc, H. et al. Tumor cell resistance to death receptor induced apoptosis through mutational inactivation of the proapoptotic Bcl2 homolog Bax. Nature Med. 8, 274–281 (2002).Shows that in DNA mismatch-repair-deficient tumour cells, Bax mutation can cause resistance to death-receptor ligands, including APO2L/TRAIL. It also shows that BAX-dependent resistance to this ligand can be circumvented by pre-exposure of the cells to chemotherapy, which upregulates DR5 and especially Bak expression.
Deng, Y., Lin, Y. & Wu, X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/Diablo. Genes Dev. 16, 33–45 (2002).
Rampino, N. et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275, 967–969 (1997).
Banner, D. W. et al. Crystal structure of the soluble human 55 kD TNF receptor–human TNFβ complex: implications for TNF receptor activation. Cell 73 431–445 (1993).
Kischkel, F. C. et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14, 5579–5588 (1995).
Kischkel, F. C. et al. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12, 611–620 (2000).
Sprick, M. R. et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12, 599–609 (2000).
Bodmer, J. L. et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nature Cell Biol. 2, 241–243 (2000).
Kischkel, F. C. et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276, 46639–46646 (2001).
Wang, J., Chun, H. J., Wong, W., Spencer, D. M. & Lenardo, M. J. Caspase-10 is an initiator caspase in death receptor signaling. Proc. Natl Acad. Sci. USA 98, 13884–13888 (2001).
Hsu, H., Shu, H. B., Pan, M. G. & Goeddel, D. V. TRADD–TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84, 299–308 (1996).
Hsu, H., Huang, J., Shu, H., Baichwal, V. & Goeddel, D. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Chinnaiyan, A. M. et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 274, 990–992 (1996).
Marsters, S. et al. Apo3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-κB. Curr. Biol. 6, 1669–1676 (1996).
Kitson, J. et al. A death-domain-containing receptor that mediates apoptosis. Nature 384, 372–375 (1996).
Pan, G. et al. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 431, 351–356 (1998).
Zhao, H. et al. Impaired c–Jun amino terminal kinase activity and T cell differentiation in death receptor 6–deficient mice.. J. Exp. Med. 194, 1441–1448 (2001).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Medscape DrugInfo
OMIM
<i>Saccharomyces</i> Genome Database
FURTHER INFORMATION
American College of Physicians
Avi Ashkenazi's lab at Genentech
Glossary
- FC DOMAIN
-
The antibody molecule can be proteolytically cleaved into two pieces — the F(ab′)2 fragment, which contains the antigen-binding activity, and the Fc domain, which carries out the effector function of the immunoglobin molecule.
- POLYHISTIDINE TAG
-
A type of epitope tag that is made up of six histidine residues (6X-His).
- EPITOPE TAG
-
A short amino-acid sequence that is added, in frame, to either end of a gene. This allows the recombinant protein to be easily detected and purified using antibodies against the tag. Commonly used tags include MYC, glutathione-S-transferase, and FLAG.
Rights and permissions
About this article
Cite this article
Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2, 420–430 (2002). https://doi.org/10.1038/nrc821
Issue Date:
DOI: https://doi.org/10.1038/nrc821
This article is cited by
-
γ-tocotrienol regulates gastric cancer by targeting notch signaling pathway
Hereditas (2023)
-
Diversity and complexity of cell death: a historical review
Experimental & Molecular Medicine (2023)
-
Pharmacokinetics and immunogenicity of eftozanermin alfa in subjects with previously-treated solid tumors or hematologic malignancies: results from a phase 1 first-in-human study
Cancer Chemotherapy and Pharmacology (2023)
-
Anti-apoptosis effect of traditional Chinese medicine in the treatment of cerebral ischemia–reperfusion injury
Apoptosis (2023)
-
Effects and mechanism of small molecule additives on recombinant protein in CHO cells
Applied Microbiology and Biotechnology (2023)