Abstract
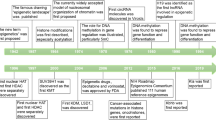
Despite advances in the prevention and management of cardiovascular disease (CVD), this group of multifactorial disorders remains a leading cause of mortality worldwide. CVD is associated with multiple genetic and modifiable risk factors; however, known environmental and genetic influences can only explain a small part of the variability in CVD risk, which is a major obstacle for its prevention and treatment. A more thorough understanding of the factors that contribute to CVD is, therefore, needed to develop more efficacious and cost-effective therapy. Application of the 'omics' technologies will hopefully make these advances a reality. Epigenomics has emerged as one of the most promising areas that will address some of the gaps in our current knowledge of the interaction between nature and nurture in the development of CVD. Epigenetic mechanisms include DNA methylation, histone modification, and microRNA alterations, which collectively enable the cell to respond quickly to environmental changes. A number of CVD risk factors, such as nutrition, smoking, pollution, stress, and the circadian rhythm, have been associated with modification of epigenetic marks. Further examination of these mechanisms may lead to earlier prevention and novel therapy for CVD.
Key Points
-
Risk of cardiovascular disease (CVD) is determined by the complex interaction of multiple genetic and environmental factors; however, most of the genetic contribution to CVD risk remains unaccounted for
-
The prenatal and postnatal environment can induce changes in gene expression that are independent of the DNA sequence and arise as a result of epigenetic mechanisms
-
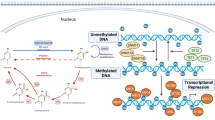
Epigenetic modifications that may have a role in increasing CVD risk include DNA methylation, histone acetylation and deacetylation, and alterations in levels of microRNAs
-
Many of the genes associated with CVD risk factors or CVD itself are known to be regulated by epigenetic mechanisms
-
The effect of environmental factors, such as diet, smoking, pollution, and stress on CVD risk may be mediated by epigenetic changes
-
Further knowledge of epigenetics might contribute to the development of novel tools for CVD prevention and therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mathers, C. D. & Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3, e442 (2006).
MacKinnon, A. U. The origin of the modern epidemic of coronary artery disease in England. J. R. Coll. Gen. Pract. 37, 174–176 (1987).
Azambuja, M. I. & Levins, R. Coronary heart disease (CHD)-—one or several diseases? Changes in the prevalence and features of CHD. Perspect. Biol. Med. 50, 228–242 (2007).
Dawber, T. R., Meadors, G. F. & Moore, F. E. Jr. Epidemiological approaches to heart disease: the Framingham Study. Am. J. Public Health Nations Health 41, 279–281 (1951).
Gersh, B. J., Sliwa, K., Mayosi, B. M. & Yusuf, S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur. Heart J. 31, 642–648 (2010).
Grundy, S. M. et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110, 227–239 (2004).
Sabatti, C. et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 41, 35–46 (2009).
Sipido, K. R. et al. Identifying needs and opportunities for advancing translational research in cardiovascular disease. Cardiovasc. Res. 83, 425–435 (2009).
Omics.org Alphabetically ordered list of omes and omics [online], (2009).
Turan, N., Katari, S., Coutifaris, C. & Sapienza, C. Explaining inter-individual variability in phenotype: is epigenetics up to the challenge? Epigenetics 5, 16–19 (2010).
Wierda, R. J., Geutskens, S. B., Jukema, J. W., Quax, P. H. & van den Elsen, P. J. Epigenetics in atherosclerosis and inflammation. J. Cell. Mol. Med. doi:10.1111/j.1582-4934201001022.x.
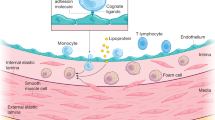
Turunen, M. P., Aavik, E. & Ylä-Herttuala, S. Epigenetics and atherosclerosis. Biochim. Biophys. Acta 1790, 886–891 (2009).
Gluckman, P. D., Hanson, M. A., Buklijas, T., Low, F. M. & Beedle, A. S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 5, 401–408 (2009).
Krause, B., Sobrevia, L. & Casanello, P. Epigenetics: new concepts of old phenomena in vascular physiology. Curr. Vasc. Pharmacol. 7, 513–520 (2009).
Ling, C. & Groop, L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 58, 2718–2725 (2009).
Mack, C. P. An epigenetic clue to diabetic vascular disease. Circ. Res. 103, 568–570 (2008).
Stöger, R. Epigenetics and obesity. Pharmacogenomics 9, 1851–1860 (2008).
Gluckman, P. D. & Hanson, M. A. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int. J. Obes. 32 (Suppl. 7), S62–S71 (2008).
Campión, J., Milagro, F. I. & Martínez, J. A. Individuality and epigenetics in obesity. Obes. Rev. 10, 383–392 (2009).
Dong, C., Yoon, W. & Goldschmidt-Clermont, P. J. DNA methylation and atherosclerosis. J. Nutr. 132, 2406S–2409S (2002).
Sharma, P. et al. Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis. 5, 1 (2006).
Stenvinkel, P. et al. Impact of inflammation on epigenetic DNA methylation—a novel risk factor for cardiovascular disease? J. Intern. Med. 261, 488–499 (2007).
Lund, G. & Zaina, S. Atherosclerosis risk factors can impose aberrant DNA methylation patterns: a tale of traffic and homocysteine. Curr. Opin. Lipidol. 20, 448–449 (2009).
Yideng, J. et al. Homocysteine-mediated expression of SAHH, DNMTs, MBD2, and DNA hypomethylation potential pathogenic mechanism in VSMCs. DNA Cell Biol. 26, 603–611 (2007).
Sharma, P. et al. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol. 27, 357–365 (2008).
Lee, M. E. & Wang, H. Homocysteine and hypomethylation. A novel link to vascular disease. Trends Cardiovasc. Med. 9, 49–54 (1999).
Castro, R., Rivera, I., Blom, H. J., Jakobs, C. & Tavares de Almeida, I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J. Inherit. Metab. Dis. 29, 3–20 (2006).
Libby, P., Okamoto, Y., Rocha, V. Z. & Folco, E. Inflammation in atherosclerosis: transition from theory to practice. Circ. J. 74, 213–220 (2010).
Pons, D. et al. Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur. Heart J. 30, 266–277 (2009).
Goyal, B. R., Patel, M. M., Soni, M. K. & Bhadada, S. V. Therapeutic opportunities of small interfering RNA. Fundam. Clin. Pharmacol. 23, 367–386 (2009).
Silvestri, P. et al. MicroRNAs and ischemic heart disease: towards a better comprehension of pathogenesis, new diagnostic tools and new therapeutic targets. Recent Pat. Cardiovasc. Drug Discov. 4, 109–118 (2009).
Mishra, P. J. & Bertino, J. R. MicroRNA polymorphisms: the future of pharmacogenomics, molecular epidemiology and individualized medicine. Pharmacogenomics 10, 399–416 (2009).
Buysschaert, I., Schmidt, T., Roncal, C., Carmeliet, P. & Lambrechts, D. Genetics, epigenetics and pharmaco-(epi)genomics in angiogenesis. J. Cell. Mol. Med. 12, 2533–2551 (2008).
Esteller, M. Epigenetics in evolution and disease. Lancet 372, S90–S96 (2008).
Waddington, C. H. Organisers and Genes (Cambridge University Press, Cambridge, 1940).
Waddington, C. H. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (1942).
Holliday, R. & Pugh, J. E. DNA modification mechanisms and gene activity during development. Science 187, 226–232 (1975).
Chen, Z. X. & Riggs, A. D. Maintenance and regulation of DNA methylation patterns in mammals. Biochem. Cell Biol. 83, 438–448 (2005).
Turner, B. M. Histone acetylation as an epigenetic determinant of long-term transcriptional competence. Cell. Mol. Life Sci. 54, 21–31 (1998).
Ghildiyal, M. & Zamore, P. D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 10, 94–108 (2009).
Filipowicz, W., Bhattacharyya, S. N. & Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 (2008).
Barringhaus, K. G. & Zamore, P. D. MicroRNAs: regulating a change of heart. Circulation 119, 2217–2224 (2009).
Katey, J. et al. miR-33 coordinates genes regulating cholesterol homeostasis. Science (in press).
Kriaucionis, S. & Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930 (2009).
Skinner, M. K., Manikkam, M. & Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21, 214–222 (2010).
Meehan, R. R. DNA methylation in animal development. Semin. Cell Dev. Biol. 14, 53–65 (2003).
Mehler, M. F. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog. Neurobiol. 86, 305–341 (2008).
Subramanian, S. & Steer, C. J. MicroRNAs as gatekeepers of apoptosis. J. Cell. Physiol. 223, 289–298 (2010).
Kwiatkowski, D. P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77, 171–192 (2005).
Krebs, J. R. The gourmet ape: evolution and human food preferences. Am. J. Clin. Nutr. 90, 707S–711S (2009).
Kelley, J. L. & Swanson, W. J. Positive selection in the human genome: from genome scans to biological significance. Annu. Rev. Genomics Hum. Genet. 9, 143–160 (2008).
Neel, J. V. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 14, 353–362 (1962).
Stöger, R. The thrifty epigenotype: an acquired and heritable predisposition for obesity and diabetes? Bioessays 30, 156–166 (2008).
Roseboom, T., de Rooij, S. & Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 82, 485–491 (2006).
Lumey, L. H. et al. Cohort profile: the Dutch Hunger Winter families study. Int. J. Epidemiol. 36, 1196–1204 (2007).
Kyle, U. G. & Pichard, C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr. Opin. Clin. Nutr. Metab. Care 9, 388–394 (2006).
Stein, A. D. et al. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am. J. Clin. Nutr. 85, 869–876 (2007).
de Rooij, S. R., Painter, R. C., Holleman, F., Bossuyt, P. M. & Roseboom, T. J. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am. J. Clin. Nutr. 86, 1219–1224 (2007).
Dover, G. J. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Trans. Am. Clin. Climatol. Assoc. 120, 199–207 (2009).
Wadhwa, P. D., Buss, C., Entringer, S. & Swanson, J. M. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 27, 358–368 (2009).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Waterland, R. A. & Michels, K. B. Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 27, 363–388 (2007).
Waterland, R. A. & Jirtle, R. L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 23, 5293–5300 (2003).
Bogdarina, I., Welham, S., King, P. J., Burns, S. P. & Clark, A. J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 100, 520–526 (2007).
Heijmans, B. T. et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA 105, 17046–17049 (2008).
Tobi, E. W. et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 18, 4046–4053 (2009).
El-Maarri, O. et al. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Hum. Genet. 122, 505–514 (2007).
Alkemade, F. E. et al. Prenatal exposure to apoE deficiency and postnatal hypercholesterolemia are associated with altered cell-specific lysine methyltransferase and histone methylation patterns in the vasculature. Am. J. Pathol. 176, 542–548 (2010).
van Straten, E. M. et al. The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R275–R282 (2010).
[No authors listed] Active and passive tobacco exposure: a serious pediatric health problem. A statement from the Committee on Atherosclerosis and Hypertension in Children, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 90, 2581–2590 (1994).
Breton, C. V. et al. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 180, 462–467 (2009).
Xue, F. & Michels, K. B. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 8, 1088–1100 (2007).
Fraga, M. F. & Esteller, M. Epigenetics and aging: the targets and the marks. Trends Genet. 23, 413–418 (2007).
Baccarelli, A. et al. Rapid DNA methylation changes after exposure to traffic particles. Am. J. Respir. Crit. Care Med. 179, 572–578 (2009).
Hoffmann, B. et al. Residential traffic exposure and coronary heart disease: results from the Heinz Nixdorf Recall Study. Biomarkers 14 (Suppl. 1), 74–78 (2009).
Launay, J. M. et al. Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS ONE 4, e7959 (2009).
Lai, C. Q. et al. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Hum. Genet. 125, 199–209 (2009).
Dodge, K. A. Practice and public policy in the era of gene-environment interactions. Novartis Found. Symp. 293, 87–97 (2008).
Kuzawa, C. W. & Sweet, E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am. J. Hum. Biol. 21, 2–15 (2009).
Mosca, L. et al. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Writing Group. Circulation 96, 2468–2482 (1997).
Abbott, R. D. et al. Joint distribution of lipoprotein cholesterol classes. The Framingham study. Arteriosclerosis 3, 260–272 (1983).
Gabory, A., Attig, L. & Junien, C. Sexual dimorphism in environmental epigenetic programming. Mol. Cell. Endocrinol. 304, 8–18 (2009).
Martino, T. A. & Sole, M. J. Molecular time: an often overlooked dimension to cardiovascular disease. Circ. Res. 105, 1047–1061 (2009).
Hermida, R. C., Ayala, D. E., Fernández, J. R. & Calvo, C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 51, 69–76 (2008).
Portaluppi, F. & Lemmer, B. Chronobiology and chronotherapy of ischemic heart disease. Adv. Drug Deliv. Rev. 59, 952–965 (2007).
Nakahata, Y., Grimaldi, B., Sahar, S., Hirayama, J. & Sassone-Corsi, P. Signaling to the circadian clock: plasticity by chromatin remodeling. Curr. Opin. Cell Biol. 19, 230–237 (2007).
Grimaldi, B., Nakahata, Y., Kaluzova, M., Masubuchi, S. & Sassone-Corsi, P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int. J. Biochem. Cell Biol. 41, 81–86 (2009).
Pegoraro, M. & Tauber, E. The role of microRNAs (miRNA) in circadian rhythmicity. J. Genet. 87, 505–511 (2008).
Rudic, R. D. & Fulton, D. J. Pressed for time: the circadian clock and hypertension. J. Appl. Physiol. 107, 1328–1338 (2009).
Hoffman, A. E. et al. CLOCK in breast tumorigenesis: genetic, epigenetic, and transcriptional profiling analyses. Cancer Res. 70, 1459–1468 (2010).
Zhang, J., Burridge, K. A. & Friedman, M. H. In vivo differences between endothelial transcriptional profiles of coronary and iliac arteries revealed by microarray analysis. Am. J. Physiol. Heart Circ. Physiol. 295, H1556–H1561 (2008).
Piekarz, R. L. et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 27, 5410–5417 (2009).
Lin, Y. C. et al. Statins increase p21 through inhibition of histone deacetylase activity and release of promoter-associated HDAC1/2. Cancer Res. 68, 2375–2383 (2008).
Mitro, N. et al. Insights in the regulation of cholesterol 7alpha-hydroxylase gene reveal a target for modulating bile acid synthesis. Hepatology 46, 885–897 (2007).
Dje N'Guessan, P. et al. Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 29, 380–386 (2009).
Fang, M., Chen, D. & Yang, C. S. Dietary polyphenols may affect DNA methylation. J. Nutr. 137, 223S–228S (2007).
Gusterson, R. J., Jazrawi, E., Adcock, I. M. & Latchman, D. S. The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J. Biol. Chem. 278, 6838–6847 (2003).
Balasubramanyam, K. et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 279, 51163–51171 (2004).
Morimoto, T. et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Invest. 118, 868–878 (2008).
Dhillon, N. et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 14, 4491–4499 (2008).
Chen, W., Bacanamwo, M. & Harrison, D. G. Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mRNA transcription. J. Biol. Chem. 283, 16293–16298 (2008).
Choi, K. C. et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 69, 583–592 (2009).
Balasubramanyam, K. et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 279, 33716–33726 (2004).
Wang, B. et al. Phosphorylation and acetylation of histone H3 and autoregulation by early growth response 1 mediate interleukin 1beta induction of early growth response 1 transcription. Arterioscler. Thromb. Vasc. Biol. 30, 536–545 (2010).
Shin, I. S. et al. Early growth response factor-1 is associated with intraluminal thrombus formation in human abdominal aortic aneurysm. J. Am. Coll. Cardiol. 53, 792–799 (2009).
Abdel-Malak, N. A., Mofarrahi, M., Mayaki, D., Khachigian, L. M. & Hussain, S. N. Early growth response-1 regulates angiopoietin-1-induced endothelial cell proliferation, migration, and differentiation. Arterioscler. Thromb. Vasc. Biol. 29, 209–216 (2009).
Oka, D. et al. The presence of aberrant DNA methylation in noncancerous esophageal mucosae in association with smoking history: a target for risk diagnosis and prevention of esophageal cancers. Cancer 115, 3412–3426 (2009).
Manolio, T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Slatkin, M. Epigenetic inheritance and the missing heritability problem. Genetics 182, 845–850 (2009).
[No authors listed] Moving AHEAD with an international human epigenome project. Nature 454, 711–715 (2008).
Acknowledgements
This work was supported by grants from the National Heart, Lung and Blood Institute (HL-54776), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK075030), and by contracts 53-K06-5-10 and 58-1950-9-001 from the USDA Department of Agricultural Research Service.
Author information
Authors and Affiliations
Contributions
J. M. Ordovás and C. E. Smith contributed to discussion of content for the article, researched data to include in the manuscript, reviewed and edited the manuscript before submission, and revised the manuscript in response to the peer-reviewers' comments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ordovás, J., Smith, C. Epigenetics and cardiovascular disease. Nat Rev Cardiol 7, 510–519 (2010). https://doi.org/10.1038/nrcardio.2010.104
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2010.104
This article is cited by
-
The contribution of the exposome to the burden of cardiovascular disease
Nature Reviews Cardiology (2023)
-
Effects of Maternal Nutrition on Oral Health in Offspring
Current Oral Health Reports (2023)
-
Why epigenetics is (not) a biosocial science and why that matters
Clinical Epigenetics (2022)
-
Offspring production of haploid spermatid-like cells derived from mouse female germline stem cells with chromatin condensation
Cell & Bioscience (2022)
-
Altered DNA methylation pattern reveals epigenetic regulation of Hox genes in thoracic aortic dissection and serves as a biomarker in disease diagnosis
Clinical Epigenetics (2021)