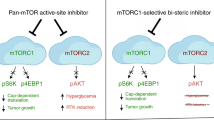
Abstract
Mammalian target of rapamycin (mTOR) is a protein kinase of the PI3K/Akt signaling pathway. Activation of mTOR in response to growth, nutrient and energy signals leads to an increase in protein synthesis, which is required for tumor development. This feature makes mTOR an attractive target for cancer therapy. First-generation mTOR inhibitors are sirolimus derivatives (rapalogs), which have been evaluated extensively in cancer patients. Everolimus and temsirolimus are already approved for the treatment of renal-cell carcinoma. Temsirolimus is also approved for the treatment of mantle-cell lymphoma. These drugs, in addition to ridaforolimus (formerly deforolimus) and sirolimus, are currently being evaluated in clinical trials of various cancers. Second-generation mTOR inhibitors are small molecules that target the kinase domain, and have also entered clinical development. Clinical trials are underway to identify additional malignancies that respond to mTOR inhibitors, either alone or in combination with other therapies. Future research should evaluate the optimal drug regimens, schedules, patient populations, and combination strategies for this novel class of agents.
Key Points
-
Mammalian target of rapamycin (mTOR) is a central regulator of cell proliferation in response to growth factors and energy stimuli
-
In some tumor types, such as renal-cell carcinoma and certain lymphomas, mTOR has a key role in tumor cell proliferation and angiogenesis
-
Temsirolimus and everolimus administered as single agents are associated with substantial improvements in patients with advanced renal-cell carcinoma, and are approved for use in this indication
-
Temsirolimus is also approved for use in mantle-cell lymphoma, where it has shown a notable improvement in progression-free survival
-
Biomarkers that assess pathway activation are being explored to identify tumor types that are sensitive to mTOR inhibition
-
Combinations of targeted therapies could improve outcomes by augmenting anti-tumor activity and overcoming mechanisms of resistance
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lane, H. A. & Breuleux, M. Optimal targeting of the mTORC1 kinase in human cancer. Curr. Opin. Cell Biol. 21, 219–229 (2009).
Abraham, R. T. & Eng, C. H. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin. Ther. Targets 12, 209–222 (2008).
Marone, R., Cmiljanovic, V., Giese, B. & Wymann, M. P. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim. Biophys. Acta 1784, 159–185 (2008).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 (2002).
Sarbassov, D. D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Copp, J., Manning, G. & Hunter, T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 69, 1821–1827 (2009).
Garcia, J. A. & Danielpour, D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol. Cancer Ther. 7, 1347–1354 (2008).
Huang, J. & Manning, B. D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 37, 217–222 (2009).
Shah, O. J., Wang, Z. & Hunter, T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14, 1650–1656 (2004).
Carracedo, A. et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 118, 3065–3074 (2008).
O'Reilly, K. E. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 (2006).
Bjornsti, M. A. & Houghton, P. J. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell 5, 519–523 (2004).
Bjornsti, M. A. & Houghton, P. J. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer 4, 335–348 (2004).
Faivre, S., Kroemer, G. & Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 5, 671–688 (2006).
Hudes, G. R. Targeting mTOR in renal cell carcinoma. Cancer 115, 2313–2320 (2009).
Grünwald, V. et al. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 62, 6141–6145 (2002).
Aguirre, D. et al. Bcl-2 and CCND1/CDK4 expression levels predict the cellular effects of mTOR inhibitors in human ovarian carcinoma. Apoptosis 9, 797–805 (2004).
deGraffenried, L. A. et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin. Cancer Res. 10, 8059–8067 (2004).
Dancey, J. in ASCO Educational Book 2000 68–75 (Lippincott Williams & Wilkins, Alexandria, Virginia, 2000).
Yu, K. et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 69, 6232–6240 (2009).
Breuleux, M. et al. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol. Cancer Ther. 8, 742–753 (2009).
Guba, M. et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat. Med. 8, 128–135 (2002).
Zhong, H., Hanrahan, C., van der Poel, H. & Simons, J. W. Hypoxia-inducible factor 1alpha and 1beta proteins share common signaling pathways in human prostate cancer cells. Biochem. Biophys. Res. Commun. 28 4, 352–356 (2001).
Zhong, H. et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60, 1541–1545 (2000).
Ballou, L. M. & Lin, R. Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 1, 27–36 (2008).
Edinger, A. L., Linardic, C. M., Chiang, G. G., Thompson, C. B. & Abraham, R. T. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 63, 8451–8460 (2003).
Sarbassov, D. D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Cloughesy, T.F. et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 5, e8 (2008).
Shor, B. et al. A new pharmacologic action of CCI-779 involves FKBP12-independent inhibition of mTOR kinase activity and profound repression of global protein synthesis. Cancer Res. 68, 2934–2943 (2008).
Foster, D. A. & Toschi, A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle 8, 1026–1029 (2009).
O'Donnell, A. et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J. Clin. Oncol. 26, 1588–1595 (2008).
Tabernero, J. et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J. Clin. Oncol. 26, 1603–1610 (2008).
Fouladi, M. et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J. Clin. Oncol. 25, 4806–4812 (2007).
Hess, G. et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 27, 3822–3829 (2009).
Ellard, S. et al. A randomized phase II study of two different schedules of RAD001C in patients with recurrent/metastatic breast cancer [abstract]. J. Clin. Oncol. 25, a3513 (2007).
Raymond, E. et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J. Clin. Oncol. 22, 2336–2347 (2004).
Geoerger, B. et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 61, 1527–1532 (2001).
Brattström, C. et al. Pharmacokinetics and safety of single oral doses of sirolimus (rapamycin) in healthy male volunteers. Ther. Drug Monit. 22, 537–544 (2000).
Filler, G., Bendrick-Peart, J. & Christians, U. Pharmacokinetics of mycophenolate mofetil and sirolimus in children. Ther. Drug Monit. 30, 138–142 (2008).
Kirchner, G. I., Meier-Wiedenbach, I. & Manns, M. P. Clinical pharmacokinetics of everolimus. Clin. Pharmacokinet. 43, 83–95 (2004).
Neuhaus, P., Klupp, J. & Langrehr, J. M. mTOR inhibitors: an overview. Liver Transpl. 7, 473–484 (2001).
Duran, I. et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur. J. Cancer 42, 1875–1880 (2006).
Gibbons, J. J. et al. The effect of CCI-779, a novel macrolide anti-tumor agent, on the growth of human tumor cells in vitro and in nude mouse xenografts in vivo [abstract]. Proc. Ame. Assoc. Cancer Res. 40, a2000 (1999).
Clackson, T. et al. Broad anti-tumor activity of AP23573, an mTOR inhibitor in clinical development [abstract]. Proc. Am. Soc. Clin. Oncol. 22, a882 (2003).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
Hudes, G. et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281 (2007).
Kapoor, A. Malignancy in kidney transplant recipients. Drugs 68 (Suppl. 1), 11–19 (2008).
Monaco, A. P. The role of mTOR inhibitors in the management of posttransplant malignancy. Transplantation 87, 157–163 (2009).
Krymskaya, V. P. & Goncharova, E. A. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle 8, 403–413 (2009).
Sampson, J. R. Therapeutic targeting of mTOR in tuberous sclerosis. Biochem. Soc. Trans. 37, 259–264 (2009).
Bissler, J. J. et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 358, 140–151 (2008).
Davies, D. M. et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N. Engl. J. Med. 358, 200–203 (2008).
Marsh, D. J. et al. Rapamycin treatment for a child with germline PTEN mutation. Nat. Clin. Pract. Oncol. 5, 357–361 (2008).
Del Bufalo, D. et al. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 66, 5549–5554 (2006).
Dal Col, J. et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood 111, 5142–5151 (2008).
Witzig, E. et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed non-hodgkin lymphoma (NHL) and Hodgkin Disease (HD) [abstract]. Haematologica 94 (Suppl. 2), a1081 (2009).
Smith, S. M. et al. Activity of single agent temsirolimus (CCI-779) in non-mantle cell non-Hodgkin lymphoma subtypes [abstract]. J. Clin. Oncol. 26, a8514 (2008).
Witzig, T. E. et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J. Clin. Oncol. 2 3, 5347–5356 (2005).
Ansell, S. M. et al. Anti-tumor activity of mTOR inhibitor temsirolimus for relapsed mantle cell lymphoma: A phase II trial in the North Central Cancer Treatment Group [abstract]. J. Clin. Oncol. 24, a2732 (2006).
Oza, A. M. et al. Molecular correlates associated with a phase II study of temsirolimus (CCI-779) in patients with metastatic or recurrent endometrial cancer--NCIC IND 160 [abstract]. J. Clin. Oncol. 2 4, a3003 (2006).
Chawla, S. P. et al. Updated results of a phase II trial of AP23573, a novel mTOR inhibitor, in patients (pts) with advanced soft tissue or bone sarcomas [abstract]. J. Clin. Oncol. 24, a9505 (2006).
Okuno, S. H. et al. A multicenter phase 2 consortium (P2C) study of the mTOR inhibitor CCI-779 in advanced soft tissue sarcomas (STS) [abstract]. J. Clin. Oncol. 24, a9504 (2006).
Duran, I. et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br. J. Cancer 95, 1148–1154 (2006).
Yao, J. C. et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J. Clin. Oncol. 26, 4311–4318 (2008).
Oza, A. M. et al. A phase II study of temsirolimus (CCI-779) in patients with metastatic and/or locally advanced recurrent endometrial cancer previously treated with chemotherapy: NCIC CTG IND 160b [abstract]. J. Clin. Oncol. 26, a5516 (2008).
Colombo, N. et al. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer [abstract]. J. Clin. Oncol. 25, a5516 (2007).
Pandya, K. J. et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500). J. Thorac. Oncol. 2, 1036–1041 (2007).
Owonikoko, T. K. et al. Phase II study of RAD001 (Everolimus) in previously treated small cell lung cancer (SCLC) [abstract]. J. Clin. Oncol. 26, a19017 (2008).
Soria, J. C. et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann. Oncol. 20, 1674–1681 (2009).
Wolpin, B. M. et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J. Clin. Oncol. 27, 193–198 (2009).
Garrido-Laguna, I. et al. Preclinical identification of biomarkers of response to mTOR inhibitors and subsequent application in a phase II trial of sirolimus in pancreatic cancer patients refractory to gemcitabine [abstract]. J. Clin. Oncol. 27, a4612 (2009).
Margolin, K. et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer 104, 1045–1048 (2005).
Rao, R. D. et al. N0377: Results of NCCTG phase II trial of the mTOR inhibitor RAD-001 in metastatic melanoma [abstract]. J. Clin. Oncol. 25, a8530 (2007).
Chang, S. M. et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest. New Drugs 23, 357–361 (2005).
Galanis, E. et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol. 2 3, 5294–5304 (2005).
Rizzieri, D. A. et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 14, 2756–2762 (2008).
Yee, K. W. et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 12, 5165–5173 (2006).
Hartford, C. M. et al. A Phase I trial to determine the safety, tolerability, and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin. Cancer Res. 15, 1428–1434 (2009).
Hidalgo, M. et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin. Cancer Res. 12, 5755–5763 (2006).
Mita, M. M. et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J. Clin. Oncol. 26, 361–367 (2008).
Ma, W. W. et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J. Clin. Oncol. 27, 2697–2704 (2009).
Cejka, D. et al. FDG uptake is a surrogate marker for defining the optimal biological dose of the mTOR inhibitor everolimus in vivo. Br. J. Cancer 100, 1739–1745 (2009).
Salvesen, H. B. et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc. Natl Acad. Sci. USA 10 6, 4834–4839 (2009).
Lane, H. The potential of mTOR inhibitors for the treatment of human cancers [abstract]. AACR Meeting Abstracts 2007, SY21–01 (2007).
Manegold, P. C. et al. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (everolimus) increases radiosensitivity in solid cancer. Clin. Cancer Res. 14, 892–900 (2008).
Murphy, J. D. et al. Inhibition of mTOR radiosensitizes soft tissue sarcoma and tumor vasculature. Clin. Cancer Res. 15, 589–596 (2009).
Nagata, Y. et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6, 117–127 (2004).
Casa, A. J., Dearth, R. K., Litzenburger, B. C., Lee, A. V. & Cui, X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front. Biosci. 13, 3273–3287 (2008).
Lu, C. H. et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin. Cancer Res. 13, 5883–5888 (2007).
La Monica, S. et al. Everolimus restores gefitinib sensitivity in resistant non-small cell lung cancer cell lines. Biochem. Pharmacol. 78, 460–468 (2009).
Reardon, D. A. et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 96, 219–230 (2010).
Kreisl, T. N. et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J. Neurooncol. 92, 99–105 (2009).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
El-Hashemite, N., Walker, V., Zhang, H. & Kwiatkowski, D. J. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 63, 5173–5177 (2003).
Fischer, P. et al. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma [abstract]. J. Clin. Oncol. 26, a16020 (2008).
Giessinger, S. et al. A phase I study with a daily regimen of the oral mTOR inhibitor RAD001 (Everolimus) plus sorafenib for patients with metastatic renal cell cancer (MRCC) [abstract]. J. Clin. Oncol. 26, a14603 (2008).
Merchan, J. R. et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: Phase I safety and activity results [abstract]. J. Clin. Oncol. 25, a5034 (2007).
Patnaik, A. et al. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies [abstract]. J. Clin. Oncol. 2 5, a3512 (2007).
Rosenberg, J. E. et al. Phase I study of sorafenib and RAD001 for metastatic clear cell renal cell carcinoma [abstract]. J. Clin. Oncol. 26, a5109 (2008).
Merchan, J. R. et al. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): Safety and activity in RTKI refractory RCC patients [abstract]. J. Clin. Oncol. 27, a5039 (2009).
Houghton, P. Targeting the IGF-1/mTOR pathway. AACR Meeting Abstracts 2008, PL05–03 (2008).
Kurmasheva, R. T., Easton, J. B. & Houghton, P. J. Combined targeting of mTOR and the insulin-like growth factor pathway. ASCO Educational Book 2008, 460–464 (2008).
McDaid, H. M. et al. Combined MEK and mTOR suppression is synergistic in human NSCLC and is mediated via inhibition of protein translation [abstract]. J. Clin. Oncol. 25, a10615 (2007).
Meier, F. et al. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death and abrogates invasive growth of melanoma cells [abstract]. J. Clin. Oncol. 26, a20033 (2008).
Carracedo, A., Baselga, J. & Pandolfi, P. P. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle 7, 3805–3809 (2008).
Atkins, M. B. et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 22, 909–918 (2004).
Amato, R. J., Jac, J., Giessinger, S., Saxena, S. & Willis, J. P. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer 115, 2438–2446 (2009).
Chan, S. et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 23, 5314–5322 (2005).
Farag, S. S. et al. Phase II trial of temsirolimus (CCI-779) in patients with relapsed or refractory multiple myeloma (MM): Preliminary results [abstract]. J. Clin. Oncol. 24, a7616 (2006).
Yee, K. W. L. et al. A phase II study of temsirolimus (CCI-779) in patients with advanced leukemias [abstract]. Blood (ASH Annual Meeting Abstracts) 104, a4523 (2004).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
J. Dancey has worked as a consultant for Wyeth.
Rights and permissions
About this article
Cite this article
Dancey, J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol 7, 209–219 (2010). https://doi.org/10.1038/nrclinonc.2010.21
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.21
This article is cited by
-
DTX3L Accelerates Pancreatic cancer Progression via FAK/PI3K/AKT Axis
Biochemical Genetics (2024)
-
Lysosomal cystine export regulates mTORC1 signaling to guide kidney epithelial cell fate specialization
Nature Communications (2023)
-
Synthetic virology approaches to improve the safety and efficacy of oncolytic virus therapies
Nature Communications (2023)
-
STRESS granule-associated RNA-binding protein CAPRIN1 drives cancer progression and regulates treatment response in nasopharyngeal carcinoma
Medical Oncology (2022)
-
A DAP5/eIF3d alternate mRNA translation mechanism promotes differentiation and immune suppression by human regulatory T cells
Nature Communications (2021)