Key Points
-
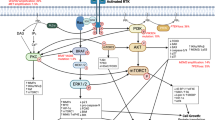
Gliomas are strikingly heterogeneous tumours in terms of their pathology and gene expression, even within a single tumour. But despite this variability, common alterations within specific cellular signal transduction pathways or cellular functions can be found in most malignant gliomas, some of which represent opportunities for therapeutic intervention.
-
This article focuses on molecularly targeted therapies for malignant glioma, in particular, those aimed at growth factor receptors (including the epidermal growth factor receptor, the vascular endothelial growth factor receptor and the platelet-derived growth factor receptor), the RAS/RAF/MAPK/ERK pathway and mammalian target of rapamycin.
-
Clinical trial results so far are presented, and key issues in the clinical development of these therapies are discussed, including drug delivery, monitoring of activity and the use of drug combinations.
Abstract
Malignant gliomas are highly lethal tumours despite maximal therapy. Traditional treatments for these cancers — which rely on nonspecific, cytotoxic approaches that generally act through damaging DNA — have a marginal impact on patient survival. However, advances in the understanding of the molecular biology underlying glioma pathogenesis have revealed abnormalities in a set of common cellular pathways and functions among the majority of these tumours that are now being targeted by novel agents in preclinical and clinical development. Such molecularly targeted agents might offer the promise of improved tumour control without substantial toxicity. Still, significant challenges in their development remain: the inability to predict tumour response and limitations of drug delivery into the tumour. Two essential aspects of glioma therapy remain to be achieved: local control of the primary tumour and blockade of tumour-cell invasion of normal brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Legler, J. M. et al. Brain and other central nervous system cancers: recent trends in incidence and mortality. J. Natl Cancer Inst. 91, 2050A–2051A (1999).
Wrensch, M., Minn, Y., Chew, T., Bondy, M. & Berger, M. S. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol. 4, 278–299 (2002).
Ron, E. et al. Tumors of the brain and nervous system after radiotherapy in childhood. N. Engl. J. Med. 319, 1033–1039 (1988).
Kleihues, P. & Cavenee, W. K. Pathology and Genetics of Tumors of the Nervous System (IARC, Lyon, 2000). Excellent overview of the pathology, epidemiology and biology of nervous system neoplasms.
Burger, P. C., Vogel, F. S. & Green, S. B. Glioblastoma multiforme and anaplastic astrocytoma: pathologic criteria and prognostic implications. Cancer 56, 1106–1111 (1985).
Bauman, G. et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int. J. Radiat. Oncol. Biol. Phys. 45, 923–929 (1999). Useful analysis of patient characteristics that heavily influence patient outcome. Clinical trials in neuro-oncology must incorporate these factors in their design.
Pignatti, F. et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. 20, 2076–2084 (2002).
Keles, G. E., Lamborn, K. R. & Berger, M. S. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J. Neurosurg. 95, 735–745 (2001).
Shaw, E. et al. Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J. Clin. Oncol. 20, 2267–2276 (2002).
Quinn, J. A. et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J. Clin. Oncol. 21, 646–651 (2003).
Scott, C. B. et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int. J. Radiat. Oncol. Biol. Phys. 40, 51–55 (1998).
Davis, F. G., McCarthy, B. J., Freels, S., Kupelian V. & Bondy, M. L. The conditional probability of survival of patients with primary malignant brain tumors. Surveillance, epidemiology, and end results (SEER) data. Cancer 85, 485–491 (1999).
Behin, A., Hoang-Xuan, K., Carpentier, A. F. & Delattre, J. Y. Primary brain tumours in adults. Lancet 361, 323–331 (2003). Overview of glioma and lymphoma management.
Sawaya, R. Extent of resection in malignant gliomas: a critical summary. J. Neurooncol. 42, 303–305 (1999).
Stewart, L. A. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359, 1011–1018 (2002).
Wallner, K. E., Galicich, J. H., Krol, G., Arbit, E. & Malkin, M. G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 16, 1405–1409 (1989).
Senger, D., Cairncross, J. G. & Forsyth, P. A. Long-term survivors of glioblastoma: statistical aberration or important unrecognized molecular subtype? Cancer J. 9, 214–221 (2003).
Smith, J. S. et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol. 18, 636–645 (2000).
Cairncross, J. G. et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendroglioma. J. Natl Cancer Inst. 90, 1473–1479 (1998).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000). A seminal review of cancer biology.
Kleihues, P. & Ohgaki, H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro-oncol. 1, 44–51 (1999).
von Deimling, A. et al. Subsets of glioblastoma multiforme defined by molecular genetic analysis. Brain Pathol. 3, 19–26 (1993).
van Meyel, D. J. et al. p53 mutation, expression, and DNA ploidy in evolving gliomas: evidence for two pathways of progression. J. Natl Cancer Inst. 86, 1011–1017 (1994).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Bogler, O., Huang, H. J. & Cavenee, W. K. Loss of wild-type p53 bestows a growth advantage on primary cortical astrocytes and facilitates their in vitro transformation. Cancer Res. 55, 2746–2751 (1995).
Malkin, D. et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250, 1233–1238 (1990).
von Deimling, A. et al. p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma. Cancer Res. 52, 2987–2990 (1992).
Sidransky, D. et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature 355, 846–847 (1992).
Rasheed, B. K. et al. Alterations of the TP53 gene in human gliomas. Cancer Res. 54, 1324–1330 (1994).
Sung, T. et al. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 10, 249–259 (2000).
Momand, J. et al. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69, 1237–1245 (1992).
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997).
Biernat, W., Kleihues, P., Yonekawa, Y. & Ohgaki, H. Amplification and overexpression of MDM2 in primary (de novo) glioblastomas. J. Neuropathol. Exp. Neurol. 56, 180–185 (1997).
Quelle, D. E., Zindy, F., Ashmun, R. A. & Sherr, C. J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Pomerantz, J. et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92, 713–723 (1998).
Honda, R. & Yasuda, H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 18, 22–27 (1999).
Nakamura, M. et al. p14ARF deletion and methylation in genetic pathways to glioblastomas. Brain Pathol. 11, 159–168 (2001).
Newcomb, E. W., Alonso, M., Sung, T. & Miller, D. C. Incidence of p14ARF gene deletion in high-grade adult and pediatric astrocytomas. Hum. Pathol. 31, 115–119 (2000).
Ichimura, K. et al. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1–S transition control gene abnormalities. Cancer Res. 60, 417–424 (2000).
Fulci, G. et al. p53 gene mutation and ink4a-arf deletion appear to be two mutually exclusive events in human glioblastoma. Oncogene 19, 3816–3822 (2000).
Sherr, C. J. Cancer cell cycles. Science 274, 1672–1677 (1996).
Biernat, W. et al. Alterations of cell cycle regulatory genes in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 94, 303–309 (1997).
Ichimura, K., Schmidt, E. E., Goike, H. M. & Collins, V. P. Human glioblastomas with no alterations of the CDKN2A (p16INK4A, MTS1) and CDK4 genes have frequent mutations of the retinoblastoma gene. Oncogene 13, 1065–1072 (1996).
Jen, J. et al. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 54, 6353–6358 (1994).
Ueki, K. et al. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 56, 150–153 (1996).
Schmidt, E. E., Ichimura, K., Reifenberger, G. & Collins, V. P. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 54, 6321–6324 (1994).
He, J., Olson, J. J. & James, C. D. Lack of p16INK4 or retinoblastoma protein (pRb), or amplification-associated overexpression of cdk4 is observed in distinct subsets of malignant glial tumors and cell lines. Cancer Res. 55, 4833–4836 (1995).
Schmidt, E. E. et al. Infrequent methylation of CDKN2A(MTS1/p16) and rare mutation of both CDKN2A and CDKN2B(MTS2/p15) in primary astrocytic tumours. Br. J. Cancer 75, 2–8 (1997).
Kapoor, G. S. & O'Rourke, D. M. Mitogenic signaling cascades in glial tumors. Neurosurgery 52, 1425–1434 (2003).
Libermann, T. A. et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 313, 144–147 (1985). Early recognition of EGFR alterations in gliomas.
Humphrey, P. A. et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 48, 2231–2238 (1988).
Ekstrand, A. J. et al. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 51, 2164–2172 (1991).
Frederick, L., Wang, X. Y., Eley, G. & James, C. D. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 60, 1383–1387 (2000).
Fujimoto, M. et al. Expression of three viral oncogenes (v-sis, v-myc, v-fos) in primary human brain tumors of neuroectodermal origin. Neurology 38, 289–293 (1988).
Nister, M. et al. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-α and their receptors in human malignant glioma cell lines. Cancer Res. 48, 3910–3918 (1988). Original report of the expression of PDGF pathway components in malignant gliomas.
Hermanson, M. et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 52, 3213–3219 (1992).
Guha, A. et al. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int. J. Cancer 60, 168–173 (1995).
Hermanson, M. et al. Association of loss of heterozygosity on chromosome 17p with high platelet-derived growth factor α receptor expression in human malignant gliomas. Cancer Res. 56, 164–171 (1996).
Gammeltoft, S. et al. Expression of two types of receptor for insulin-like growth factors in human malignant glioma. Cancer Res. 48, 1233–1237 (1988).
Rosen, E. M. et al. Scatter factor expression and regulation in human glial tumors. Int. J. Cancer. 67, 248–255 (1996).
Plate, K. H., Breier, G., Weich, H. A. & Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359, 845–848 (1992).
Berkman, R. A. et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J. Clin. Invest. 91, 153–159 (1993).
Constam, D. B. et al. Differential expression of transforming growth factor-β1,-β2, and -β3 by glioblastoma cells, astrocytes, and microglia. J. Immunol. 148, 1404–1410 (1992).
Kjellman, C. et al. Expression of TGF-β isoforms, TGF-β receptors, and SMAD molecules at different stages of human glioma. Int. J. Cancer 89, 251–258 (2000).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nature Rev. Cancer 3, 11–22 (2003).
Burgart, L. J., Robinson, R. A., Haddad, S. F. & Moore, S. A. Oncogene abnormalities in astrocytomas: EGF-R gene alone appears to be more frequently amplified and rearranged compared with other protooncogenes. Mod. Pathol. 4, 183–186 (1991).
Guha, A. et al. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene 15, 2755–2765 (1997).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase–Akt pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Choe, G. et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 63, 2742–2746 (2003).
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997). This and the following reports first identified PTEN as an important novel tumour suppressor gene.
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997).
Lin, H. et al. Allelic deletion analyses of MMAC/PTEN and DMBT1 loci in gliomas: relationship to prognostic significance. Clin. Cancer Res. 4, 2447–2454 (1998).
Sano, T. et al. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res. 59, 1820–1824 (1999).
Raffel, C. et al. Analysis of oncogene and tumor suppressor gene alterations in pediatric malignant astrocytomas reveals reduced survival for patients with PTEN mutations. Clin. Cancer Res. 5, 4085–4090 (1999).
Myers, M. P. et al. PTEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl Acad. Sci. USA 94, 9052–9057 (1997).
Tamura, M. et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 (1998).
Myers, M. P. et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl Acad. Sci. USA 95, 13513–13518 (1998). This and the following two reports outlined surprising roles for PTEN regulating phospholipids as crucial in its tumour-suppressive functions.
Wu, X. et al. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl Acad. Sci. USA 95, 15587–15591 (1998).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Su, J. D., Mighto, L. D., Donner, D. B. & Durden, D. L. PTEN and phosphatidylinositol 3′-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 63, 3585–3592 (2003).
Abe, T. et al. PTEN decreases in vivo vascularization of experimental gliomas in spite of proangiogenic stimuli. Cancer Res. 63, 2300–2305 (2003).
Koul, D. et al. Suppression of matrix metalloproteinase-2 gene expression and invasion in human glioma cells by MMAC/PTEN. Oncogene 20, 6669–6678 (2001).
Kuriyama, H. et al. Prognostic significance of an apoptotic index and apoptosis/proliferation ratio for patients with high-grade astrocytomas. Neuro-oncol. 4, 179–186 (2002).
Heesters, M. A., Koudstaal, J., Go, K. G. & Molenaar, W. M. Analysis of proliferation and apoptosis in brain gliomas: prognostic and clinical value. J. Neurooncol. 44, 255–266 (1999).
Trepel, M. et al. Chemosensitivity of human malignant glioma: modulation by p53 gene transfer. J. Neurooncol. 39, 19–32 (1998).
Vogelbaum, M. A. et al. Transfection of C6 glioma cells with the bax gene and increased sensitivity to treatment with cytosine arabinoside. J. Neurosurg. 88, 99–105 (1998).
Nagane, M. et al. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 60, 847–853 (2000).
Fulda, S., Wick, W., Weller, M. & Debatin, K. M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nature Med. 8, 808–815 (2002).
Shingu, T. et al. Synergistic augmentation of antimicrotubule agent-induced cytotoxicity by a phosphoinositide 3-kinase inhibitor in human malignant glioma cells. Cancer Res. 63, 4044–4047 (2003).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Hahn, W. C. Role of telomeres and telomerase in the pathogenesis of human cancer. J. Clin. Oncol. 21, 2034–2043 (2003).
Morin, G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 59, 521–529 (1989).
Meyerson, M. et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 (1997). Initial report of the cloning of the human telomerase catalytic subunit that is expressed in many cancers.
Kim, N. -W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Langford, L. A. et al. Telomerase activity in human brain tumours. Lancet 346, 1267–1268 (1995).
Hiraga, S. et al. Telomerase activity and alterations in telomere length in human brain tumors. Cancer Res. 58, 2117–2125 (1998).
Hakin-Smith, V. et al. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet 361, 836–838 (2003).
Yamaguchi, F. et al. Anti-telomerase therapy suppressed glioma proliferation. Oncol. Rep. 6, 773–776 (1999).
Holland, E. C., Hively, W. P., DePinho, R. A. & Varmus, H. E. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 12, 3675–3685 (1998). One of several papers delineating an elegant malleable model of malignant glioma formation.
Holland, E. C. et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nature Genet. 25, 55–57 (2000).
Dai, C. et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 15, 1913–1925 (2001).
Xiao, A. et al. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell 1, 157–168 (2002). PTEN loss might function in a tissue-specific fashion to increase tumour malignancy.
Weiss, W. A. et al. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 63, 1589–1595 (2003).
Bachoo, R. M. et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1, 269–277 (2002). Report suggesting that differentiated astrocytes might be driven to dedifferentiate and transform into gliomas.
Reilly, K. M. et al. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nature Genet. 26, 109–113 (2000).
Vogel, K. S. et al. Mouse tumor model for neurofibromatosis type 1. Science 286, 2176–2179 (1999).
Ding, H. et al. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 61, 3826–3836 (2001).
Ding, H. et al. Oligodendrogliomas result from the expression of an activated mutant epidermal growth factor receptor in a RAS transgenic mouse astrocytoma model. Cancer Res. 63, 1106–1113 (2003).
Rich, J. N. et al. A genetically tractable model of human glioma formation. Cancer Res. 61, 3556–3560 (2001).
Sonoda, Y. et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 61, 4956–4960 (2001).
Yancopoulos, G. D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Bergers, G. et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111, 1287–1295 (2003). Elegant delineation of the relative contributions of vascular components to sustained tumour angiogenesis.
Lesniak, M. S., Langer, R. & Brem, H. Drug delivery to tumors of the central nervous system. Curr. Neurol. Neurosci. Rep. 1, 210–216 (2001).
Pardridge, W. M. Molecular biology of the blood–brain barrier. Methods Mol. Med. 89, 385–399 (2003).
Kenney, J. et al. Measurement of blood–brain barrier permeability in a tumor model using magnetic resonance imaging with gadolinium-DTPA. Magn. Reson. Med. 27, 68–75 (1992).
Boucher, Y. et al. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br. J. Cancer 75, 829–836 (1997).
Bendell, J. C. et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977 (2003).
Reardon, D. A. et al. Phase II trial of murine (131)I-labeled anti-tenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 20, 1389–1397 (2002).
Williams, J. A. et al. Preirradiation osmotic blood-brain barrier disruption plus combination chemotherapy in gliomas: quantitation of tumor response to assess chemosensitivity. Adv. Exp. Med. Biol. 331, 273–284 (1993).
Matsukado, K., Sugita, M. & Black, K. L. Intracarotid low dose bradykinin infusion selectively increases tumor permeability through activation of bradykinin B2 receptors in malignant gliomas. Brain Res. 792, 10–15 (1998).
Bobo, R. H. et al. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl Acad. Sci. USA 91, 2076–2080 (1994).
Aboody, K. S. et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl Acad. Sci USA 97, 12846–12851 (2000).
Pietras, K. et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 61, 2929–2934 (2001).
Sampson, J. H. et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-α and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J. Neurooncol. 65, 27–35 (2003).
Debinski, W. et al. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin. Cancer Res. 1, 1253–1258 (1995).
Weber, F. et al. Safety, tolerability, and tumor response of IL4–Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 64, 125–137 (2003).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001). Seminal proof of principle of clinical efficacy of small-molecule signal transduction inhibitors in cancer therapy.
Joensuu, H. et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N. Engl. J. Med. 344, 1052–1056 (2001).
Pandita, A. et al. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer 39, 29–36 (2004).
Weiss, W. A. et al. Neuropathology of genetically engineered mice: consensus report and recommendations from an international forum. Oncogene 21, 7453–7463 (2002).
Gossmann, A. et al. Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J. Magn. Reson. Imaging 15, 233–240 (2002).
Grossman, S. A. et al. Increased 9-aminocamptothecin dose requirements in patients on anticonvulsants. NABTT CNS Consortium. The new approaches to brain tumor therapy. Cancer Chemother. Pharmacol. 42, 118–126 (1998).
De Smet, P. Drug therapy: Herbal remedies. N. Engl. J. Med. 347, 2046–2056 (2002).
Ranson, M. et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J. Clin. Oncol. 20, 2240–2250 (2002).
Felsher, D. W. Cancer revoked: Oncogenes as therapeutic targets. Nature Rev. Cancer 3, 375–379 (2003).
Wong, A. J. et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc. Natl Acad. Sci. USA 84, 6899–6903 (1987).
Sugawa, N., Ekstrand, A. J., James, C. D. & Collins V. P. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc. Natl Acad. Sci. USA 87, 8602–8606 (1990).
Huang, H. S. et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Biol. Chem. 272, 2927–2935 (1997).
Lund-Johansen, M. et al. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 50, 6039–6044 (1990).
Goldman, C. K. et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol. Biol. Cell 4, 121–133 (1993).
Nishikawa, R. et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl Acad. Sci. USA 91, 7727–7731 (1994).
Nagane, M. et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 56, 5079–5086 (1996).
Shinojima, N. et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 63, 6962–6970 (2003).
Wikstrand, C. J. & Bigner, D. D. Prognostic applications of the epidermal growth factor receptor and its ligand, transforming growth factor-α. J. Natl Cancer Inst. 90, 799–801 (1998).
Miyaji, K. et al. Effect of tyrphostin on cell growth and tyrosine kinase activity of epidermal growth factor receptor in human gliomas. J. Neurosurg. 81, 411–419 (1994).
Han, Y. et al. Tyrphostin AG 1478 preferentially inhibits human glioma cells expressing truncated rather than wild-type epidermal growth factor receptors. Cancer Res. 56, 3859–3861 (1996).
Penar, P. L., Khoshyomn, S., Bhushan, A. & Tritton, T. R. Inhibition of epidermal growth factor receptor-associated tyrosine kinase blocks glioblastoma invasion of the brain. Neurosurgery 40, 141–151 (1997).
Rich, J. N. et al. Phase II trial of gefitinib in recurrent glioblastoma. J. Clin. Oncol. 22, 133–142 (2004). A report on the outcome of the first completed trial of EGFR tyrosine kinase inhibitors in recurrent glioblastoma, which showed modest efficacy.
Lieberman, F. S. et al. Phase I–II study of ZD-1839 for recurrent malignant gliomas and meningiomas progressing after radiation therapy. Proc. Am. Soc. Clin. Oncol. 22, 105 (2003).
Prados, M. et al. Phase I study of OSI–774 alone or with temozolomide in patients with malignant glioma. Proc. Am. Soc. Clin. Oncol. 22, 99 (2003).
Vogelbaum, M. A. et al. Initial experience with the EGFR tyrosine kinase inhibitor Tarceva (OSI-774) for single-agent therapy of recurrent/progressive glioblastoma multiforme. Abstracts of the Society of Neuro-Oncology Eighth Annual Meeting. Neuro-oncol. 5, 356 (2003).
Mishima, K. et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 61, 5349–5354 (2001).
Sampson, J. H. et al. Unarmed, tumor specific monoclonal antibody effectively treats brain tumors. Proc. Natl Acad. Sci. USA 97, 7503–7508 (2000).
Grossman, S. A. et al. Toxicity, efficacy, and pharmacology of suramin in adults with recurrent high-grade gliomas. J. Clin. Oncol. 19, 3260–3266 (2001).
Yu, J., Ustach, C. & Kim, H. R. Platelet-derived growth factor signaling and human cancer. J. Biochem. Mol. Biol. 36, 49–59 (2003).
Lokker, N. A., Sullivan, C. M., Hollenbach, S. J., Israel, M. A. & Giese, N. A. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands might play a role in the development of brain tumors. Cancer Res. 62, 3729–3735 (2002).
Uhrbom, L., Hesselager, G., Nister, M. & Westermark, B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 58, 5275–5279 (1998).
Kilic, T. et al. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 60, 5143–5150 (2000).
Shawver, L. K. et al. Inhibition of platelet-derived growth factor-mediated signal transduction and tumor growth by N-[4-(trifluoromethyl)-phenyl]5-methylisoxazole-4-carboxamide. Clin. Cancer Res. 3, 1167–1177 (1997).
Vassbotn, F. S. et al. Activated platelet-derived growth factor autocrine pathway drives the transformed phenotype of a human glioblastoma cell line. J. Cell. Physiol. 158, 381–389 (1994).
Strawn, L. M. et al. Inhibition of glioma cell growth by a truncated platelet-derived growth factor-β receptor. J. Biol. Chem. 269, 21215–21222 (1994).
Behl, C. et al. Autocrine growth regulation in neuroectodermal tumors as detected with oligodeoxynucleotide antisense molecules. Neurosurgery 33, 679–684 (1993).
Shamah, S. M., Stiles, C. D. & Guha, A. Dominant-negative mutants of platelet-derived growth factor revert the transformed phenotype of human astrocytoma cells. Mol. Cell. Biol. 13, 7203–7212 (1993).
Wen, P. Y. et al. Phase I study of STI571 (Gleevec) for patients with recurrent malignant gliomas and meningiomas (NABTC 99–08). Proc. Am. Soc. Clin. Oncol. 21, 73A (2002).
Dresemann, G. STI 571/hydroxyurea in progressive, pretreated glioblastoma (GB) patients (pts.). Proc. Am. Soc. Clin. Oncol. 22, 116 (2003).
Fong, T. A. et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 59, 99–106 (1999).
Laird, A. D. et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 60, 4152–4160 (2000).
Yung, W. K. A. et al. A phase I trial of single-agent PTK 787/ZK 222584 (PTK/ZK), an oral VEGFR tyrosine kinase inhibitor, in patients with recurrent glioblastoma multiforme. Proc. Am. Soc. Clin. Oncol. 22, 99 (2003).
Reardon, D et al. Preliminary phase I trial results: PTK787/ZK222584 (PTK/ZK), an oral VEGF tyrosine kinase inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme. Abstracts of the Society of Neuro-Oncology Eighth Annual Meeting. Neuro-oncol. 5, 355 (2003).
Sebti, S. M. & Der, C. J. Searching for the elusive targets of farnesyltransferase inhibitors. Nature Rev. Cancer 3, 945–951 (2003).
Kohl, N. E. et al. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science 260, 1934–1937 (1993).
Bredel, M., Pollack, I. F., Freund, J. M., Hamilton, A. D. & Sebti, S. M. Inhibition of Ras and related G-proteins as a therapeutic strategy for blocking malignant glioma growth. Neurosurgery 43, 124–131 (1998).
Cloughesy, T. F. et al. Phase II trial of R115777 (Zarnestra) in patients with recurrent glioma not taking enzyme inducing antiepileptic drugs (EIAED): a North American Brain Tumor Consortium (NABTC) report. Proc. Am. Soc. Clin. Oncol. 21, 80A (2002).
Kuhn, J. G. et al. Phase I trial of R115777 (Zarnestra) in patients with recurrent malignant glioma taking enzyme inducting antiepileptic drugs (EIAED). A North American Brain Tumor Consortium (NABTC) report. Proc. Am. Soc. Clin. Oncol. 21, 86A (2002).
Shamji, A. F., Nghiem, P. & Schreiber, S. L. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol. Cell 12, 271–280 (2003).
Abraham, R. T. Identification of TOR signaling complexes: more TORC for the cell growth engine. Cell 111, 9–12 (2002).
Singh, K., Sun, S. & Vezina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. IV. Mechanism of action. J. Antibiot. (Tokyo) 32, 630–645 (1979).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Houchens, D. P., Ovejera, A. A., Riblet, S. M. & Slagel, D. E. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur. J. Cancer Clin. Oncol. 19, 799–805 (1983).
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001). An important indicator of a molecular predictor of a targeted therapy.
Chang, S. et al. Phase II/pharmacokinetic study of CCI-779 in recurrent glioblastoma multiforme. Abstracts of the Society of Neuro-Oncology Eighth Annual Meeting. Neuro-oncol. 5, 349 (2003).
Rich, J. N. The role of transforming growth factor-β in primary brain tumors. Front. Biosci. 8, E245–E260 (2003).
Fakhrai, H. et al. Eradication of established intracranial rat gliomas by transforming growth factor β antisense gene therapy. Proc. Natl Acad. Sci. USA 93, 2909–2914 (1996).
Stauder, G. M. et al. A TGF-β2 specific antisense oligonucleotide (AP12009) as continuous intratumoral treatment of recurrent high-grade glioma patients: A clinical phase I/II extension study. Proc. Am. Soc. Clin. Oncol. 22, 109 (2003).
Rich, J. N., Zhang, M., Datto, M. B., Bigner, D. D. & Wang, X. F. Transforming growth factor-β-mediated p15(INK4B) induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. J. Biol. Chem. 274, 35053–35058 (1999).
Hjelmeland, M. D. et al. SB431542, a small molecule transforming growth factor-α-receptor antagonist, inhibits human glioma xenograft malignant phenotype. 2003 AACR-NCI–EORTC Int. Conf. Molecular Targets Cancer Therapeutics A C136 (2003).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876–880 (2001).
Li, B. et al. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res. 63, 7443–7450 (2003).
Chakravarti, A., Loeffler, J. S. & Dyson, N. J. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 62, 200–207 (2002).
Klingler-Hoffmann, M., Bukczynska, P. & Tiganis, T. Inhibition of phosphatidylinositol 3-kinase signaling negates the growth advantage imparted by a mutant epidermal growth factor receptor on human glioblastoma cells. Int. J. Cancer 105, 331–339 (2003).
Baker, C. H., Solorzano, C. C. & Fidler, I. J. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 62, 1996–2003 (2002).
Goudar, R. et al. Combination therapy of inhibitors of the epidermal growth factor/ vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. 2003 AACR-NCI–EORTC Int. Conf. Molecular Targets Cancer Therapeutics A A88 (2003).
Rao, R. D. et al. Synergistic inhibition of glioma cell growth upon combination therapy with the mTOR inhibitor, rapamycin, and the epidermal growth factor receptor inhibitor, EKI-785. Proc. Am. Assoc. Cancer Res. 44, A718 (2003).
Nagane, M. et al. Human glioblastoma xenografts overexpressing a tumor-specific mutant epidermal growth factor receptor sensitized to cisplatin by the AG1478 tyrosine kinase inhibitor. J. Neurosurg. 95, 472–479 (2001).
Chakravarti, A. et al. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 62, 4307–4315 (2002).
Geng, L. et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 61, 2413–2419 (2001).
Giaccone, G. et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a Phase III trial — INTACT 1. J. Clin. Oncol. 22, 777–784 (2004).
Herbst, R. S. et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a Phase III trial — INTACT 2. J. Clin. Oncol. 22, 785–794 (2004).
Tremont-Lukats, I. W. & Gilbert, M. R. Advances in molecular therapies in patients with brain tumors. Cancer Control 10, 125–137 (2003).
Rao, R. D., Uhm, J. H., Krishnan, S. & James, C. D. Genetic and signaling pathway alterations in glioblastoma: relevance to novel targeted therapies. Front. Biosci. 8, E270–E280 (2003).
Mischel, P. S. & Cloughesy, T. F. Targeted molecular therapy of GBM. Brain Pathol. 13, 52–61 (2003).
Newton, H. B. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 1: Growth factor and Ras signaling pathways. Expert Rev. Anticancer Ther. 3, 595–614 (2003).
Reilly, K. M. & Jacks, T. Genetically engineered mouse models of astrocytoma: GEMs in the rough? Semin. Cancer Biol. 11, 177–191 (2001).
Acknowledgements
The authors would like to thank H. Friedman, J. Quinn, C. Wikstrand, X.-F. Wang, D. Reardon, J. Vredenburgh, R. McLendon and T. Curran for helpful discussions. This work was supported in part by funding from the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure and the Southeastern Brain Tumor Foundation. This work was supported by National Institutes of Health grants, General Clinical Research Center Program, National Center for Research Resources, a National Cancer Institute grant, Finding Cures for Glioblastoma grants, and a grant from the Pediatric Brain Tumor Foundation of the United States.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J. R. has seved as a paid consultant for Novartis.
Glossary
- TUMOUR XENOGRAFT
-
Tumour specimens can be grown in immunocompromised rodents to provide tumour models with many of the complexities of human tumours.
- CLINICAL ENDPOINT
-
A characteristic or variable that reflects how a patient feels or functions, or how long a patient survives.
- BIOMARKER
-
A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention.
Rights and permissions
About this article
Cite this article
Rich, J., Bigner, D. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov 3, 430–446 (2004). https://doi.org/10.1038/nrd1380
Issue Date:
DOI: https://doi.org/10.1038/nrd1380
This article is cited by
-
The potential role of N7-methylguanosine (m7G) in cancer
Journal of Hematology & Oncology (2022)
-
Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation
Journal of Nanobiotechnology (2021)
-
Biomimetic nanomedicine toward personalized disease theranostics
Nano Research (2021)
-
LncRNA CCAT2 promotes angiogenesis in glioma through activation of VEGFA signalling by sponging miR-424
Molecular and Cellular Biochemistry (2020)
-
In Vitro Reconstruction of Brain Tumor Microenvironment
BioChip Journal (2019)