Key Points
-
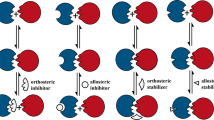
PDZ domains are involved in the recruitment and interaction of proteins, and aid the formation of protein scaffolds and signalling networks. This is achieved by sequence-specific binding between a PDZ domain in one protein and a PDZ motif in another protein.
-
Targeting the PDZ domain of proteins with peptides or small molecules is therefore of interest as a way to interfere with protein interactions in disease. PICK1, a ubiquitous protein that contains a PDZ domain, is used as an example to discuss approaches, inherent challenges and outstanding questions relating to drug discovery for these targets.
-
PICK1 was first reported to interact with protein kinase C, and has since been implicated in the regulation of a variety of proteins involved in normal physiological processes, such as synaptic transmission and plasticity, sound stimulation and neuropathic pain sensitization. PICK1 is also putatively involved in neurodegenerative and neurological diseases, such as stroke and schizophrenia, and is also now being implicated in cancer.
-
One advantage of targeting PDZ domains is the potential to design drugs that are specific for a particular PDZ interaction. The most well-established method of interfering with PDZ interactions is the use of blocking peptides specific to the PDZ domain sequence. These serve as tools for studying the role of PDZ in protein interactions, but also have potential as therapeutics if issues such as peptide delivery and degradation can be overcome.
-
The development of small molecules that bind directly to PDZ domains or allosterically induce conformational change that hides the PDZ domain is desirable. Progress in this field has been limited but should now improve through the mapping of PDZ domains and the availability of X-ray crystallography data
-
A significant challenge in this field is to delineate the complex protein networks that PDZ domains are involved in. It is possible that certain PDZ interactions only occur in particular cell-specific contexts or stages of development, providing an opportunity for differential regulation. PDZ knockout models will continue to shed some light on this concept.
Abstract
Modulating protein–protein interactions involved in disease pathways is an attractive strategy for developing drugs, but remains a challenge to achieve. One approach is to target certain domains within proteins that mediate these interactions. One example of such a domain is the PDZ domain, which is involved in interactions between many different proteins in a variety of cellular contexts. Because PDZ domains have well-defined binding sites, they are promising targets for drug discovery. However, there is still much to learn about the function of these domains before drugs targeting PDZ interactions can become a reality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Arkin, M. R. & Wells, J. A. Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nature Rev. Drug Discov. 3, 301–317 (2004).
Berg, T. Modulation of protein–protein interactions with small organic molecules. Angew. Chem. Int. Ed. Engl. 42, 2462–2481 (2003).
Fischer, P. M. & Lane, P. L. Small-molecule inhibitors of the p53 suppressor HDM2: have protein–protein interactions come of age as drug targets? Trends Pharmacol. Sci. 25, 343–346 (2004).
Ponting, C. P. et al. PDZ domains: targeting signalling molecules to submembranous sites. Bioessays 19, 469–479 (1997).
Kim, E. & Sheng, M. PDZ domain proteins of synapses. Nature Rev. Neurosci. 10, 771–781 (2004).
Fanning, A. S. & Anderson, J. M. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J. Clin. Invest. 103, 767–772 (1999).
Nourry, C., Grant, S. G. & Borg, J. P. PDZ domain proteins: plug and play! Sci. STKE 179, RE7 (2003).
Collingridge, G. L. & Isaac, J. T. Functional roles of protein interactions with AMPA and kainate receptors. Neurosci. Res. 47, 3–15 (2003).
Henley, J. M. Protein interactions implicated in AMPA receptor trafficking: a clear destination and an improving route map. Neurosci. Res. 45, 243–254 (2003).
Cho, K. -O. et al. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discslarge tumor suppressor protein. Neuron 9, 929–942 (1992).
Woods, D. F. & Bryant, P. J. The discs–large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66, 451–464 (1991).
Itoh, M. et al. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J. Cell Biol. 121, 491–502 (1993).
Hillier, B. J. et al. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS–syntrophin complex. Science 284, 812–815 (1999).
Songyang, Z. et al. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275, 73–77 (1997).
Staudinger, J. et al. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J. Cell Biol. 128, 263–271 (1995). First paper to report the isolation and identification of PICK1 as a protein interacting with PKCα.
Staudinger, J. et al. Specific interaction of the PDZ domain protein PICK1 with the COOH terminus of protein kinase C-α. J. Biol. Chem. 272, 32019–32024 (1997).
Dev, K. K. et al. The protein kinase C α binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology 38, 635–644 (1999).
Xia, J. et al. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22, 179–187 (1999). Together with reference 17, this first reports that PICK1 interacts with the AMPA receptor subunit Glu 2.
Boudin, H. et al. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron 28, 485–497 (2000). Together with references 35 and 36, simultaneously reported the interaction between PICK1 and mGlu 7.
Boudin, H. & Craig, A. M. Molecular determinants for PICK1 synaptic aggregation and mGluR7a receptor coclustering: role of the PDZ, coiled-coil, and acidic domains. J. Biol. Chem. 276, 30270–30276 (2001).
Hanley, J. G. et al. NSF ATPase and α-/β-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron 34, 53–67 (2002). The authors provide the first description of an interaction between PICK1 and SNAP.
Takeya, R. et al. Interaction of the PDZ domain of human PICK1 with class I ADP-ribosylation factors. Biochem. Biophys. Res. Commun. 267, 149–155 (2000). Demonstrates that PICK1 interacts with ARFs.
Peter, B. J. et al. BAR Domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 302, 495–499 (2004).
Tarricone, C. et al. The structural basis of Arfaptin mediated cross-talk between Rac and Arf signalling pathways. Nature 411, 215–219 (2001).
Perez, J. L. et al. PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J. Neurosci. 21, 5417–5428 (2001).
Wang, W. L. et al. PICK1: an anchoring protein that specifically targets PKCα to mitochondria selectivity upon serum stimulation in NIH 3T3 cells. J. Biol. Chem. 278, 37705–37712 (2003).
Lin, W. J. et al. Mitogen-stimulated TIS21 protein interacts with a protein-kinase-Cα-binding protein rPICK1. Biochem. J. 354, 635–643 (2001). First report of a PICK1 interaction with TIS21.
Jourdi, H. et al. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev. Biol. 263, 216–230 (2003)
Kim, A. R. et al. Phosphorylation of 46-kDa protein of synaptic vesicle membranes is stimulated by GTP and Ca2+/calmodulin. Exp. Mol. Med. 34, 434–443 (2002).
Dev, K. K. et al. Regulation of mGlu(7) receptors by proteins that interact with the intracellular C-terminus. Trends Pharmacol. Sci. 22, 355–361 (2001).
Meyer, G, The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology 47, 724–733 (2004). Provides evidence of an interaction between PICK1 and neuroligin.
Hirbec, H. et al. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J. Biol. Chem. 277, 15221–15224 (2002).
Barnes, G. N. & Slevin, J. T. Ionotrpoic glutamate receptor biology: effect on synaptic connectivity and function in neurological disease. Curr. Med. Chem. 10, 2059–2072 (2003).
Hirbec, H. et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37, 625–638 (2003). An interaction between PICK1 and kainate receptors is described.
Dev, K. K. et al. PICK1 interacts with and regulates PKC phosphorylation of mGluR7. J. Neurosci. 20, 7252–7257 (2000).
El Far, O. et al. Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. Eur. J. Neurosci. 12, 4215–4221 (2000).
Dong, H. et al. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386, 279–284 (1997).
Srivastava, S. et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron 21, 581–591 (1998).
Enz, R. & Croci, C. Different binding motifs in the metabotropic glutamate receptor type 7b for Filamin-A, PP1C, PICK1 and Syntenin allow the formation of multimeric protein complexes. Biochem. J. 372, 183–191 (2003). PICK1 suggested interaction between syntenin and PICK1.
Perroy, J. et al. PICK1 is required for the control of synaptic transmission by the metabotropic glutamate receptor 7. EMBO J. 21, 2990–2999 (2002).
Stowell, J. N. & Craig, A. M. Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic carboxy-terminal domains. Neuron 22, 525–536 (1999).
McInvale, A. C. et al. Immunolocalization of PICK1 in the ascending auditory pathways of the adult rat. J. Comp. Neurol. 450, 382–394 (2002).
Garry, E. M. et al. Specific involvement in neuropathic pain of AMPA receptors and adapter proteins for the GluR2 subunit. Mol. Cell. Neurosci. 24, 10–22 (2003).
Himanen, J. P. & Nikolov, D. B. Eph signaling: a structural view. Trends Neurosci. 26, 46–51 (2003).
Torres, R. et al. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 21, 1453–1463 (1998). Eph receptors were the first receptors found to interact with PICK.
Cowen, C. A. et al. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26, 417–430 (2000). The first interaction of PICK1 aquaporins and anion exchangers is described here.
Penzes, P. et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho–GEF kalirin. Neuron 37, 263–274 (2003). PICK1 is suggested to interact with Kalirin and is linked to EphB receptors.
Williams, M. E. et al. Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J. Neurosci. 23, 11279–11288 (2003). The interaction between UNC5H and PICK1 is first described.
Julin-Bastard, F. et al. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD-95/DLG/ZO-1 domain proteins. J. Biol. Chem. 276, 15256–15263 (2001). First report of an interaction between PICK1 and ErbB2/HER2.
Dziedzic, B. et al. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J. Neurosci. 23, 915–926 (2003).
Excoffon, K. J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J. Cell Sci. 117, 4401–4409 (2004). The authors demonstrate that PICK1 interacts with CAR.
Klapper, L. N. et al. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv. Cancer Res. 77, 25–79 (2000).
Torres, G. E. et al. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30, 121–134 (2001). Evidence that PICK1 interacts with monoamine transporters.
Gainetdinov, R. R. & Caron, M. G. Monoamine transporters: from genes to behavior. Annu. Rev. Pharmacol. Toxicol. 43, 261–84 (2003).
Torres, G. E. et al. Plasma membrane monoamine transporters: structure, regulation and function. Nature Rev. Neurosci. 4, 13–25 (2003).
Bengel, D. et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymeth-amphetamine ('Ecstasy') in serotonin transporter-deficient mice. Mol. Pharmacol. 53, 649–655 (1998).
Giros, B. et al. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–712 (1996).
Xu, F. et al. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nature Neurosci. 3, 465–471 (2000).
Bjerggaard, C. Surface targeting of the dopamine transporter involves discrete epitopes in the distal C terminus but does not require canonical PDZ domain interactions. J. Neurosci. 24, 7024–7036 (2004).
Hong, C. J. Association study of PICK1 rs3952 polymorphism and schizophrenia. Neuroreport 15, 1965–1967 (2004).
Leonard, A. S. et al. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc. Natl Acad. Sci. 100, 2029–2034 (2003).
Duggan, A. et al. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J. Biol. Chem. 277, 5203–5208 (2002). This paper together with ref 63 and 64 first revealed the interaction between PICK1 and BNaC channels.
Hruska-Hageman, A. M. et al. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem. J. 361, 443–450 (2002).
Baron, A. et al. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J. Biol. Chem. 277, 50463–50468 (2002).
Deval, E. et al. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J. Biol. Chem. 279, 19531–19539 (2004).
Ferro, E. S. et al. Intracellullar peptides as putative natural regulators of protein interactions. J. Neurochem. (in the press).
Daw, M. I. et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28, 873–886 (2000).
Nishimune, A. et al. NSF binding to GluR2 regulates synaptic transmission. Neuron 21, 87–97 (1998).
Li, P. et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nature Neurosci. 2, 972–977 (1999).
Xia, J. et al. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 28, 499–510 (2000).
Chung, H. J. et al. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci. 20, 7258–7267 (2000).
Kim, C. H. et al. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl Acad. Sci. USA 98, 11725–11730 (2001).
Chung, H. J. et al. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300, 1751–1755 (2003).
Matsuda, S. et al. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J. Neurochem. 73, 1765–1768 (1999).
Lin, S. H. et al. The carboxyl terminus of the prolactin-releasing peptide receptor interacts with PDZ domain proteins involved in α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor clustering. Mol. Pharmacol. 60, 916–923 (2001). These authors were first to show that PICK1 can interact with PrRP receptors.
Terashima, A. et al. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J. Neurosci. 24, 5381–5390 (2004).
Nagai, Y. et al. Prevention of polyglutamine oligomerization and neurodegeneration by the peptide inhibitor QBP1 in Drosophila. Hum. Mol. Genet. 12, 1253–1259 (2003).
El-Agnaf, O. M. et al. A strategy for designing inhibitors of α-synuclein aggregation and toxicity as a novel treatment for Parkinson's disease and related disorders. FASEB J. 18, 1315–1317 (2004).
Pawson, T. & Nash, P. Assembly of cell regulatory systems through protein interaction domains. Science 300, 445–452 (2003).
Schlessinger, J. & Lemmon, M. A. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 191, RE12 (2003).
Lemmon, M. A. Phosphoinositide recognition domains. Traffic 4, 201–213 (2003).
Joliot, A. & Prochiantz, A. Transduction peptides: from technology to physiology. Nature Cell Biol. 6, 189–196 (2004).
Iwakura, Y. et al. N-methyl-D-aspartate-induced α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor down-regulation involves interaction of the carboxyl terminus of GluR2/3 with Pick1. Ligand-binding studies using Sindbis vectors carrying AMPA receptor decoys. J. Biol. Chem. 276, 40025–40032 (2001).
Ehrengruber, M. U. et al. Gene transfer into neurons from hippocampal slices: comparison of recombinant Semliki Forest virus, adenovirus, adeno-associated virus, lentivirus, and measles virus. Mol. Cell. Neurosci. 17, 855–871 (2001).
Morris, M. C. et al. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nature Biotechnol. 19, 1173–1176 (2001).
Aarts, M. et al. Treatment of ischemic brain damage by perturbing NMDA receptor–PSD-95 protein interactions. Science 298, 846–850 (2002).
Fujii, N. et al. A selective irreversible inhibitor targeting a PDZ protein interaction domain. J. Am. Chem. Soc. 125, 12074–12075 (2003).
Harris, B. Z. et al. Role of electrostatic interactions in PDZ domain ligand recognition. Biochemistry 42, 2797–2805 (2003).
Laboto, M. N. & Rabbitts, T. H. Intracellular antibodies and challenges facing their use as therapeutic agents. Trends Mol. Med. 9, 390–396 (2003).
Clayton, J. The silent treatment. Nature 431, 599–605 (2004).
Shi, S. et al. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001).
Migaud, M. et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 (1998).
Komiyama, N. H. et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J. Neurosci. 22, 9721–9732 (2002).
Bezprozvanny, I. & Maximov, A. Classification of PDZ domains. FEBS Lett. 509, 457–462 (2001).
Daniels, D. L. et al. Crystal structure of the hCASK PDZ domain reveals the structural basis of class II PDZ domain target recognition. Nature Struct. Biol. 5, 317–325 (1998).
Toogood, P. L. Inhibition of protein–protein association by small molecules: approaches and progress. J. Med. Chem. 45, 1543–1558 (2002).
Osten, P. et al. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron 27, 313–325 (2000).
Dev, K. K. et al. The PDZ domain of PICK1 differentially accepts protein kinase C-α and GluR2 as interacting ligands. J. Biol. Chem. 279, 41393–41397 (2004).
Novak, K. A. P. et al. Investigation of the PDZ domain ligand binding site using chemically modified peptides. Bioorg. Med. Chem. Lett. 12, 2471–2474 (2002).
Zhang, M. & Wang, W. Organization of signaling complexes by PDZ-domain scaffold proteins. Acc. Chem. Res. 36, 530–538 (2003).
Ferrer, M. et al. A PDZ domain-based detection system for enzymatic assays. Anal. Biochem. 301, 207–216 (2002).
Burette, A. et al. Differential cellular and subcellular localization of the AMPA receptor-binding protein and glutamate receptor-interacting protein. J. Neurosci. 21, 495–503 (2001).
Wyszynski, M. et al. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J. Neurosci. 19, 6528–6537 (1999).
Beneyto, M. & Meador-Woodruff, J. H. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J. Comp. Neurol. 468, 530–554 (2004).
Passafaro, M. et al. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nature Neurosci. 4, 917–926 (2001).
Boeda, B. Myosin, VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 21, 6689–6699 (2002).
Bladt, F. Epidermolysis bullosa and embryonic lethality in mice lacking the multi-PDZ domain protein GRIP1. Proc. Natl Acad. Sci. USA 99, 6816–6821 (2002).
Acknowledgements
I am grateful to J. F. Cryan for critical reading of this review. I wish to thank S. Manghani for help with artwork. My thanks to R. Kuhn and D. Hoyer for continued support.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
K.D. is an employee of Novartis.
Glossary
- COILED-COIL MOTIF
-
A protein structural domain that mediates subunit oligomerization. Coiled coils contain between two and five helices that twist around each other to form a supercoil.
- SPINE-LIKE STRUCTURES
-
A particular morphology of a dendritic structure. It is a morphological extension from the dendrite that has a long shaft and a mushroom-like head.
Rights and permissions
About this article
Cite this article
Dev, K. Making protein interactions druggable: targeting PDZ domains. Nat Rev Drug Discov 3, 1047–1056 (2004). https://doi.org/10.1038/nrd1578
Issue Date:
DOI: https://doi.org/10.1038/nrd1578
This article is cited by
-
The adaptor protein PICK1 targets the sorting receptor SorLA
Molecular Brain (2022)
-
Targeting PDZ domains as potential treatment for viral infections, neurodegeneration and cancer
Biology Direct (2021)
-
miR-615-3p promotes the epithelial-mesenchymal transition and metastasis of breast cancer by targeting PICK1/TGFBRI axis
Journal of Experimental & Clinical Cancer Research (2020)
-
Modifying PTEN recruitment promotes neuron survival, regeneration, and functional recovery after CNS injury
Cell Death & Disease (2019)
-
Structure function relations in PDZ-domain-containing proteins: Implications for protein networks in cellular signalling
Journal of Biosciences (2018)