Key Points
-
TNF family members have a multitude of crucial roles in the immune response, and numerous companies have invested millions of dollars to develop therapeutic drugs that target many of these molecules, including TNF, CD40-L, RANK-L, TRAIL and CD30-L.
-
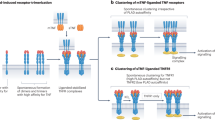
Two novel TNF family members were discovered several years ago through expressed sequence tag (EST) database searches: APRIL (a proliferation-inducing ligand) and BLyS (B-lymphocyte stimulator), which is also well known as BAFF (B-cell activating factor of the TNF family). It was subsequently shown that both ligands bind to two TNF-R family members, TACI (transmembrane activator and CAML interactor and BCMA (B-cell maturation antigen), and that BLyS also specifically binds to another TNF-R family member, BAFF-R. After a long search for a third TNF-R-like receptor that specifically binds APRIL but not BLyS, two groups recently demonstrated that APRIL also binds to heparan sulfate proteoglycans (HSPG).
-
Although the biological functions of BLyS have been more readily discerned than those of APRIL, especially from studies of transgenic and knockout mice, many recently published experiments have provided more clues about the normal and pathogenic functions of APRIL.
-
APRIL is produced by many of the same cell types that express BLyS, including monocytes, dendritic cells, macrophages and T cells, but is also produced (unlike BLyS) by various carcinomas.
-
Importantly, APRIL is also aberrantly expressed by malignant B cells (normal B cells do not express BLyS or APRIL), and by various cells within the B-cell tumour micro-environment, including 'nurse-like' cells derived from B-chronic lymphocytic leukaemia patients and osteoclasts and stromal cells in the bone marrow from multiple myeloma patients. Becuase these malignant B cells also express at least one of the three BLyS/APRIL receptors (TACI, BCMA and/or BAFF-R), and inhibition of BLyS or APRIL enhances the apoptosis of these tumour cells, many groups have proposed the existence of an autocrine and/or paracrine survival loop mediated by BLyS and APRIL.
-
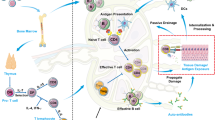
Some of the normal functions of APRIL are thought to be: co-stimulating antigen-activated B cells; increasing B-cell antigen presentation function via BCMA; enabling isotype switching in B cells; and augmenting plasma cell survival, and possibly affecting plasma cell trafficking. In addition, APRIL may co-stimulate pre-activated T cells, although the data supporting this function are still rather contentious.
-
APRIL and BLyS levels are elevated in the sera of patients suffering from a variety of autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome and multiple sclerosis. Additionally, it has recently been discovered that certain mutations in TACI in common variable immunodeficiency (CVID) and IgA deficiency (IgAD) patients lead to impaired APRIL binding and consequent inhibition of isotype switching.
-
Based on these and other findings linking APRIL and BLyS to autoimmune disease, immunodeficiency and various cancers, seven biotechnology companies have invested in the development of at least four therapeutic molecules, including a neutralizing anti-BLyS monoclonal antibody and two soluble receptors (BAFF-R and TACI), that target BLyS alone, or BLyS and APRIL together.
Abstract
Since their discovery in 1998, the two TNF family members APRIL and BLyS/BAFF have received increasing attention. In addition to regulating normal B-cell development and immune responses, these molecules might be crucial in a diverse set of diseases, including autoimmunity and cancer. Although more has been published about the general biology of BLyS/BAFF than that of APRIL, many recent articles have described novel APRIL biology. Here we focus on APRIL, exploring its normal and pathological functions, and comparing the therapeutic molecules currently under development that target BLyS/BAFF alone, or APRIL and BLyS/BAFF together.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bodmer, J. L., Schneider, P. & Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 (2002).
Hahne, M. et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med. 188, 1185–1190 (1998). The first publication describing the discovery of APRIL.
Shu, H. B., Hu, W. H. & Johnson, H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J. Leukoc. Biol. 65, 680–683 (1999).
Kelly, K., Manos, E., Jensen, G., Nadauld, L. & Jones, D. A. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 60, 1021–1027 (2000).
Moore, P. A. et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285, 260–263 (1999).
Schneider, P. et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 189, 1747–1756 (1999).
Mukhopadhyay, A., Ni, J., Zhai, Y., Yu, G. L. & Aggarwal, B. B. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J. Biol. Chem. 274, 15978–15981 (1999).
Gross, J. A. et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404, 995–999 (2000).
von Bulow, G. U. & Bram, R. J. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science 278, 138–141 (1997).
Gras, M. P. et al. BCMAp: an integral membrane protein in the Golgi apparatus of human mature B lymphocytes. Int. Immunol. 7, 1093–1106 (1995).
Thompson, J. S. et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293, 2108–2111 (2001).
Yan, M. et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr. Biol. 11, 1547–1552 (2001).
Lentz, V. M., Hayes, C. E. & Cancro, M. P. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J. Immunol. 160, 3743–3747 (1998).
Ingold, K. et al. Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 201, 1375–1383 (2005).
Hendriks, J. et al. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 12, 637–648 (2005). References 14 and 15 describe the discovery that, in addition to binding TACI and BCMA, APRIL also binds to HSPG.
Roschke, V. et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169, 4314–4321 (2002). The first publication describing the existence of BLyS/APRIL heterotrimers (HT) and demonstrating that HT are present at elevated levels in patients with various autoimmune diseases.
Kalled, S. L., Ambrose, C. & Hsu, Y. M. The biochemistry and biology of BAFF, APRIL and their receptors. Curr. Dir. Autoimmun. 8, 206–242 (2005).
Mackay, F., Sierro, F., Grey, S. T. & Gordon, T. P. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr. Dir. Autoimmun. 8, 243–265 (2005).
Kalled, S. L. The role of BAFF in immune function and implications for autoimmunity. Immunol. Rev. 204, 43–54 (2005).
Ng, L. G., Mackay, C. R. & Mackay, F. The BAFF/APRIL system: life beyond B lymphocytes. Mol. Immunol. 42, 763–772 (2005).
Schneider, P. The role of APRIL and BAFF in lymphocyte activation. Curr. Opin. Immunol. 17, 282–289 (2005).
Zola, H. et al. CD molecules 2005: human cell differentiation molecules. Blood (2005).
Pradet-Balade, B. et al. An endogenous hybrid mRNA encodes TWE-PRIL, a functional cell surface TWEAK-APRIL fusion protein. EMBO J. 21, 5711–5720 (2002).
Wallweber, H. J., Compaan, D. M., Starovasnik, M. A. & Hymowitz, S. G. The crystal structure of a proliferation-inducing ligand, APRIL. J. Mol. Biol. 343, 283–290 (2004).
Lopez-Fraga, M., Fernandez, R., Albar, J. P. & Hahne, M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2, 945–951 (2001).
Nardelli, B. et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204 (2001).
Kolfschoten, G. M., Pradet-Balade, B., Hahne, M. & Medema, J. P. TWE-PRIL; a fusion protein of TWEAK and APRIL. Biochem. Pharmacol. 66, 1427–1432 (2003).
Yan, M. et al. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nature Immunol. 1, 37–41 (2000).
Marsters, S. A. et al. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr. Biol. 10, 785–788 (2000).
Wu, Y. et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J. Biol. Chem. 275, 35478–35485 (2000).
Yu, G. et al. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nature Immunol. 1, 252–256 (2000).
Liu, Y. et al. Ligand-receptor binding revealed by the TNF family member TALL-1. Nature 423, 49–56 (2003).
Hymowitz, S. G. et al. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J. Biol. Chem. 280, 7218–7227 (2005).
Gordon, N. C. et al. BAFF/BLyS receptor 3 comprises a minimal TNF receptor-like module that encodes a highly focused ligand-binding site. Biochemistry 42, 5977–5983 (2003).
Kim, H. M. et al. Crystal structure of the BAFF-BAFF-R complex and its implications for receptor activation. Nature Struct. Biol. 10, 342–348 (2003).
Patel, D. R. et al. Engineering an APRIL-specific B cell maturation antigen. J. Biol. Chem. 279, 16727–16735 (2004).
Mackay, F. & Ambrose, C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 14, 311–324 (2003).
Rennert, P. et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 192, 1677–1184 (2000).
Schneider, P. et al. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187, 1205–1213 (1998).
Holler, N. et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol. Cell Biol. 23, 1428–1440 (2003).
Bishop, G. A. & Hostager, B. S. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 14, 297–309 (2003).
Couchman, J. R. Syndecans: proteoglycan regulators of cell-surface microdomains? Nature Rev. Mol. Cell Biol. 4, 926–937 (2003).
Day, E. S. et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry 44, 1919–1931 (2005).
Litinskiy, M. B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature Immunol. 5, 822–829 (2002).
Craxton, A., Magaletti, D., Ryan, E. J. & Clark, E. A. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101, 4464–4471 (2003).
Moreaux, J. et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 106, 1021–1030 (2005). These authors demonstrate that APRIL is produced by bone marrow stromal cells and osteoclasts, and discuss how this and other findings support a role for APRIL in normal plasma cell maintenance, and in multiple myeloma cell survival.
Ohata, J. et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J. Immunol. 174, 864–870 (2005).
Deshayes, F. et al. Abnormal production of the TNF-homologue APRIL increases the proliferation of human malignant glioblastoma cell lines via a specific receptor. Oncogene 23, 3005–3012 (2004).
Novak, A. J., Bram, R. J., Kay, N. E. & Jelinek, D. F. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood 100, 2973–2979 (2002). The first of many publications (including the following six references) demonstrating that malignant B cells aberrantly express BLyS and APRIL, and that inhibiting these ligands using soluble TACI-Ig or BCMA-Ig receptors or neutralizing mAbs can induce apoptosis of various tumor cells (multiple myeloma, CLL, NHL).
Kern, C. et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 103, 679–688 (2004).
Novak, A. J. et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood 104, 2247–2253 (2004).
Novak, A. J. et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood 103, 689–694 (2004).
He, B. et al. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J. Immunol. 172, 3268–3279 (2004).
Moreaux, J. et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 103, 3148–3157 (2004).
Nishio, M. et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1α. Blood 106, 1012–1020 (2005).
Viau, M. & Zouali, M. B-lymphocytes, innate immunity, and autoimmunity. Clin. Immunol. 114, 17–26 (2005).
Ng, L. G. et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J. Immunol. 173, 807–817 (2004).
Laabi, Y. et al. The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res. 22, 1147–1154 (1994).
Seyler, T. M. et al. BLyS and APRIL in rheumatoid arthritis. J. Clin. Invest. 115, 3083–3092 (2005).
Ye, Q. et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur. J. Immunol. 34, 2750–2759 (2004).
Stein, J. V. et al. APRIL modulates B and T cell immunity. J. Clin. Invest. 109, 1587–1598 (2002). First publication describing the phenotype of APRIL transgenic mice.
Castigli, E. et al. Impaired IgA class switching in APRIL-deficient mice. Proc. Natl Acad. Sci. USA 101, 3903–3908 (2004). This and reference 68 describe APRIL deficient (knockout) mice.
Avery, D. T. et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112, 286–297 (2003).
O'Connor, B. P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 (2004). References 63 and 64 demonstrate a role for BLyS and APRIL in mediating plasmablast and plasma cell survival.
Yang, M. et al. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell-activating factor of the TNF family, induces antigen presentation in B cells. J. Immunol. 175, 2814–2824 (2005).
Hatzoglou, A. et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-κB, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 165, 1322–1330 (2000).
Bonci, D., Hahne, M., Felli, N., Peschle, C. & De Maria, R. Potential role of APRIL as autocrine growth factor for megakaryocytopoiesis. Blood 104, 3169–3172 (2004).
Varfolomeev, E. et al. APRIL-deficient mice have normal immune system development. Mol. Cell Biol. 24, 997–1006 (2004).
Planelles, L. et al. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell 6, 399–408 (2004).
Gross, J. A. et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: impaired B cell maturation in mice lacking BLyS. Immunity 15, 289–302 (2001).
Schneider, P. et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J. Exp. Med. 194, 1691–1697 (2001).
von Bulow, G. U., van Deursen, J. M. & Bram, R. J. Regulation of the T-independent humoral response by TACI. Immunity 14, 573–582 (2001).
Yan, M. et al. Activation and accumulation of B cells in TACI-deficient mice. Nature Immunol. 2, 638–643 (2001).
Seshasayee, D. et al. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 18, 279–288 (2003).
Xu, S. & Lam, K. P. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 21, 4067–4074 (2001).
Schiemann, B. et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293, 2111–2114 (2001).
Shulga-Morskaya, S. et al. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J. Immunol. 173, 2331–2341 (2004).
Landers, C. D., Chelvarajan, R. L. & Bondada, S. The role of B cells and accessory cells in the neonatal response to TI-2 antigens. Immunol Res. 31, 25–36 (2005).
Castigli, E. et al. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201, 35–39 (2005).
Jabara, H. et al. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity 17, 265–276 (2002).
Koyama, T. et al. Raised serum APRIL levels in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 64, 1065–1067 (2005). This paper, and many others listed below, demonstrate altered levels of APRIL and/or BLyS in various autoimmune disorders.
Stohl, W. et al. Inverse association between circulating APRIL levels and serological and clinical disease activity in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 63, 1096–1103 (2004).
Koyama, T. et al. A novel polymorphism of the human APRIL gene is associated with systemic lupus erythematosus. Rheumatology (Oxford) 42, 980–985 (2003).
Ramanujam, M. et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J. Immunol. 173, 3524–3534 (2004).
Odendahl, M. et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165, 5970–5979 (2000).
Tan, S. M. et al. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 48, 982–992 (2003).
Cheema, G. S., Roschke, V., Hilbert, D. M. & Stohl, W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 44, 1313–1319 (2001).
Wang, H. et al. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nature Immunol. 2, 632–637 (2001).
Groom, J. et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J. Clin. Invest. 109, 59–68 (2002).
Mariette, X. et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann. Rheum. Dis. 62, 168–171 (2003).
Jonsson, M. V., Szodoray, P., Jellestad, S., Jonsson, R. & Skarstein, K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren's syndrome. J. Clin. Immunol. 25, 189–201 (2005).
Uccelli, A., Aloisi, F. & Pistoia, V. Unveiling the enigma of the CNS as a B-cell fostering environment. Trends Immunol. 26, 254–259 (2005).
Thangarajh, M. et al. Increased levels of APRIL (A Proliferation-Inducing Ligand) mRNA in multiple sclerosis. J. Neuroimmunol. 167, 210–214 (2005).
Thangarajh, M., Gomes, A., Masterman, T., Hillert, J. & Hjelmstrom, P. Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. J. Neuroimmunol. 152, 183–190 (2004).
Salzer, U. et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nature Genet. 37, 820–828 (2005).
Castigli, E. et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nature Genet. 37, 829–834 (2005). References 95 and 96 describe mutations in TACI found in CVID and IgAD patients that lead to impaired APRIL binding and consequent inhibition of isotype switching.
Roth, W. et al. APRIL, a new member of the tumor necrosis factor family, modulates death ligand-induced apoptosis. Cell Death Differ. 8, 403–410 (2001).
Okano, H. et al. Functional expression of a proliferation-related ligand in hepatocellular carcinoma and its implications for neovascularization. World J. Gastroenterol. 11, 4650–4654 (2005).
Craxton, A., Draves, K. E., Gruppi, A. & Clark, E. A. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J. Exp. Med. 202, 1363–1374 (2005).
Foger, N., Marhaba, R. & Zoller, M. Involvement of CD44 in cytoskeleton rearrangement and raft reorganization in T cells. J. Cell Sci. 114, 1169–1178 (2001).
Baker, K. P. et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 48, 3253–3265 (2003).
Pelletier, M. et al. Comparison of soluble decoy IgG fusion proteins of BAFF-R and BCMA as antagonists for BAFF. J. Biol. Chem. 278, 33127–33133 (2003).
Ruben, S. M. Antibodies against tumor necrosis factor delta (APRIL). Patent. US 2003/0059862 A1 (2003).
Kelley, R. F. & Patel, D. Variants of the extracellular domain of BCMA and uses thereof. Patent. WO 2005/075511 A1 (2005).
Acknowledgements
Many thanks to M. Moore, B. Fox, M. Kelley, C. Ostrander, and P. Shea for the design, expression, characterization, and purification of ZZ APRIL, to B. Harder and J. Ellsworth for providing the data shown in Fig. 2, and to K. Lewis, S. Rene and S. McMillan for performing the Biacore analyses with ZZ APRIL and BLyS described in Table 2. Thanks also to Z. Zhang, and to S. Peano and R. Ponce for sharing unpublished results. We also thank G. Vieira for artwork, and G. McKnight, M. Bernard, A. Ching, and D. Louden for assistance with literature research. Finally, we are grateful to our colleagues at Serono and ZGEN for providing helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.D. and J.G. are employees of ZymoGenetics, Inc. and have stock options in the company. A.N. and S.A. receive some research funding from ZymoGenetics as part of a research agreement.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Cluster of Differentiation nomenclature
HGS press release describing results from Phase 2 clinical trial with LymphoStat-B in SLE
Human Genome Organization nomenclature
ZGEN press release describing results from Phase 1b clinical trial with TACI-Ig in RA
Glossary
- Expressed sequence tag
-
(EST). A partial, short (200–500 base pairs) sequence from either end of a cDNA clone (that is, from a gene that has been expressed in some tissue or at some stage of development) that can be mapped, by a combination of genetic mapping procedures, to a unique locus in the genome and which serves to identify that gene locus. A database of ESTs can be helpful for picking protein-coding sequences out of a long stretch of DNA, or for providing a larger context for very short sequences.
- Heparan sulphate proteoglycans
-
(HSPGs). Ubiquitous macromolecules associated with the cell surface and extracellular matrix of a wide range of cells of vertebrate and invertebrate tissues, which are essential cofactors in cell–matrix adhesion processes, in cell–cell recognition systems, and in receptor–growth factor interactions.
- Surface plasmon resonance (Biacore)
-
A biophysical technique used to measure the binding interactions and kinetics of very small amounts of a target protein. A ligand is immobilized on a special chip, a solution of the target molecule flows across the chip and the progress of binding is then measured by optical instruments.
- Chronic lymphocytic leukaemia
-
(CLL). A common leukaemia characterized by a neoplastic proliferation of well-differentiated and mature lymphocytes (usually B cells) that is manifested by the progressive accumulation of these cells in the blood, bone marrow and lymphatic tissues.
- Germinal centre
-
A discrete area within the lymph node and spleen containing rapidly dividing B cells that respond to antigen, along with the antigen-specific helper T cells that helped activate them. Germinal centre B cells can undergo somatic hypermutation and isotype switching, and give rise to both effector and memory B cells bearing high-affinity antibodies.
- Class switch recombination
-
(CSR; also known as isotype switch). The shift of a B cell or its progeny from the secretion of antibody of one isotype or class of antibody with the same V regions but a different heavy-chain constant region and, therefore, of a different isotype.
- Immune complex
-
An antigen bound to antibody; levels of immune complexes are elevated in many autoimmune disorders.
- Non-Hodgkin's lymphoma
-
(NHL). A group of more than 29 types of lymphomas characterized by cancerous growth of B or T cells, excluding those characterized by Hodgkin's disease, and which are classified by cell type and rate of growth (aggressive or indolent).
- Nurse-like cell
-
(NLC). Cells that derive from a subset of blood CD14+ mononuclear cells from patients with CLL, which differentiate in vitro in the presence of CLL (but not normal) B cells, and can protect CLL B cells from apoptosis.
Rights and permissions
About this article
Cite this article
Dillon, S., Gross, J., Ansell, S. et al. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov 5, 235–246 (2006). https://doi.org/10.1038/nrd1982
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd1982
This article is cited by
-
Role of telitacicept in the treatment of IgA nephropathy
European Journal of Medical Research (2023)
-
Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis
Advances in Rheumatology (2022)
-
Genetic and molecular biology of systemic lupus erythematosus among Iranian patients: an overview
Autoimmunity Highlights (2021)
-
An integrative network analysis framework for identifying molecular functions in complex disorders examining major depressive disorder as a test case
Scientific Reports (2021)
-
B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies
Nature Reviews Neurology (2021)