Key Points
-
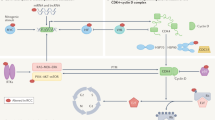
Mammalian target of rapamycin (mTOR) is a key kinase that acts as a master switch of cellular catabolism, anabolism, proliferation, cell-cycle control, autophagy, angiogenesis and apoptosis. Human malignancies display a plethora of molecular abnormalities involving phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and/or the PI3K/AKT/mTOR pathway.
-
Rapamycin, a potent mTOR inhibitor, was initially developed as an immunosuppressive agent that, unlike cyclosporine, decreases the risk of post-transplant lymphoproliferative disorders. Both rapamycin itself and rapamycin derivatives (CCI-779, RAD001 and AP23573) have demonstrated antiproliferative and antitumour effects in preclinical models and clinical malignancies.
-
CCI-779 when given weekly as a single agent was the first-in-class mTOR inhibitor to demonstrate survival improvement as compared with interferon in patients with poor-prognosis advanced renal cell carcinoma.
-
Translational studies performed during clinical trials identified molecular markers — p-S6K1 and p-4EBP1 — that were used to monitor biological effects of mTOR inhibitors.
-
Resistance to rapamycin derivatives might result from a lack of AKT activation and/or deficient apoptosis pathways (such as BCL2 expression), and useful biomarkers to predict such therapeutic responses are yet to be discovered.
-
Multitargeting approaches combining other targeted therapy, hormone therapy and/or chemotherapy might improve the therapeutic potential of mTOR derivatives.
Abstract
Mammalian target of rapamycin (mTOR) is a kinase that functions as a master switch between catabolic and anabolic metabolism and as such is a target for the design of anticancer agents. The most established mTOR inhibitors — rapamycin and its derivatives — showed long-lasting objective tumour responses in clinical trials, with CCI-779 being a first-in-class mTOR inhibitor that improved the survival of patients with advanced renal cell carcinoma. This heralded the beginning of extensive clinical programmes to further evaluate mTOR inhibitors in several tumour types. Here we review the clinical development of this drug class and look at future prospects for incorporating these agents into multitarget or multimodality strategies against cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vignot, S., Faivre, S., Aguirre, D. & Raymond, E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann. Oncol. 16, 525–537 (2005).
Larue, L. & Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24, 7443–7454 (2005).
Gschwendt, M. Protein kinase Cδ. Eur. J. Biochem. 259, 555–564 (1999).
Steinberg, S. F. Distinctive activation mechanisms and functions for protein kinase Cδ. Biochem. J. 384, 449–459 (2004).
Fang, J. Y. & Richardson, B. C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 6, 322–327 (2005).
Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8, 179–183 (2005).
Castedo, M., Ferri, K. F. & Kroemer, G. Mammalian target of rapamycin (mTOR): pro- and anti-apoptotic. Cell Death Differ. 9, 99–100 (2002).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002). An important review with most references that emphasize PI3K/AKT functions and implications in malignant tumours.
Inoki, K., Corradetti, M. N. & Guan, K. L. Dysregulation of the TSC–mTOR pathway in human disease. Nature Genet. 37, 19–24 (2005).
Johannessen, C. M. et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc. Natl Acad. Sci. USA. 102, 8573–8578 (2005).
Luo, Z., Saha, A. K., Xiang, X. & Ruderman, N. B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 26, 69–76 (2005).
Shaw, R. J. et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91–99 (2004).
Lee, L. et al. Efficacy of a rapamycin analog (CCI-779) and IFN-γ in tuberous sclerosis mouse models. Genes Chromosomes Cancer 42, 213–227 (2005).
Thomas, G. & Hall, M. N. TOR signaling and control of control of cell growth. Curr. Opin. Cell Biol. 9, 782–787 (1997).
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000).
Guertin, D. A. & Sabatini, D. M. An expanding role for mTOR in cancer. Trends Mol. Med. 11, 353–361 (2005).
Martin, D. E. & Hall, M. N. The expanding TOR signaling network. Curr. Opin. Cell Biol. 17, 158–166 (2005).
Scott, P. H. et al. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc. Natl Acad. Sci. USA 95, 7772–7777 (1998).
Nave, B T. et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344, 427–431 (1999).
Fry, M. J. Phosphoinositide 3-kinase signaling in breast cancer: how big a role might it play? Breast Cancer Res. 3, 304–312 (2001).
Hu, Q. et al. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 268, 100–102 (1995).
Lee, A. V. & Yee, D. Insulin-like growth factors and breast cancer. Biomed. Pharmacother. 49, 415–421 (1995).
Scheid, M. P. & Woodgett, J. R. Phosphatidylinositol 3′ kinase signaling in mammary tumorigenesis. J. Mammary Gland Biol. Neoplasia 6, 83–99 (2001).
Hankinson, S. E. et al. Circulating concentrations of insulin-like growth factor–I and risk of breast cancer. Lancet 351, 1393–1396 (1998).
Smith, G. D., Gunnell, D. & Holly, J. Cancer and insulin-like growth factor-I. A potential mechanism linking the environment with cancer risk. BMJ 321, 847–848 (2000).
Pollack, M. Insulin like growth factor physiology and cancer risk. Eur. J. Cancer 36, 1224–1228 (2000).
Yu, H. & Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl Cancer Inst. 92, 1472–1489 (2000).
Resnicoff, M. & Baserga, R. The role of the insulin-like growth factor–I receptor in transformation and apoptosis. Ann. NY Acad. Sci. 842, 76–81 (1998).
Cheng, J. Q. et al. Akt2, a putative oncogene encoding a member of a subfamily of serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl Acad. Sci. USA 89, 9267–9271 (1992).
Cheng, J. Q. et al. Amplification of AKT 2 in human pancreatic cells and inhibition of Akt 2 expression and tumorigenicity by antisense RNA. Proc. Natl Acad. Sci. USA 93, 3636–3641 (1996).
Bellacosa, A. et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 64, 280–285 (1995).
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001). This paper describes the crucial role of PTEN and PTEN deletions as well as the effects of mTOR inhibitors using in vivo models.
Brunn, G. J. et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 (1997).
Hara, K. et al. Regulation of eIF-4E BP1 phosphorylation by mTOR. J. Biol. Chem. 272, 26457–26463 (1997).
Chung, J. et al. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature 370, 71–75 (1994).
Gingras, A. C. et al. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12, 502–513 (1998).
Brunn, G. J. et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 (1997).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR actions. Cell 110, 177–189 (2002).
Kim, D.-H. et al. MTOR interacts with raptor to form a nutriement –sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Pain, V. M. Initiation of protein synthesis in eukaryotic cells. Eur. J. Biochem. 236, 747–771 (1996).
Proud, C. G. & Denton, R M. Molecular mechanism for the control of translation by insulin. Biochem. J. 328, 329–341 (1997).
Sonenberg, N. & Gingras, A. C. The m RNA 5′ cap-binding protein eIF4E and control of cell growth. Curr. Opin. Cell Biol. 10, 268–275 (1998).
Smith, M. R. et al. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 2, 648–654 (1990).
Rousseau, D. et al. The eIF4E-binding protein 1 and 2 are negative regulators of cell growth. Oncogene 13, 2415–2420 (1996).
Easton, J. B., Kurmasheva, R. T. & Houghton, P. J. IRS-1: auditing the effectiveness of mTOR inhibitors. Cancer Cell 9, 153–155 (2006).
Mendez, R. et al. Stimulation of protein synthesis, eukaryotic translation initiation factor 4E phosphorylation and PHAS-I phosphorylation by insulin requires insulin receptor substrate 1 and phosphatidyl inositol 3 kinase. Mol. Cell Biol. 16, 5991–6001 (1996).
Rosenwald, I. B. et al. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J. Biol. Chem. 270, 21176–21180 (1995).
Rousseau, D. et al. Translation initiation of ornithine decarboxylase and nuceocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc. Natl Acad. Sci. USA 93, 1065–1070 (1996).
Berretta, L. et al. Rapamycin blocks the phos-phorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EBMO J. 15, 658–664 (1996).
Graves, L. M. et al. c-AMP –and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc. Natl Acad. Sci. USA 92, 7222–7226 (1995).
Lin, T. A. et al. Control of PHAS-I by insulin in 3T3-L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin — sensitive and mitogen-activated protein kinase-independent pathway. J. Biol. Chem. 270, 18531–18538 (1995).
Dilling, B. D. et al. 4E-binding proteins, the suppressors of eukaryotic initiation factor 4E, are down-regulated in cells with acquired or intrinsic resistance to rapamycin. J. Biol. Chem. 277, 13907–13917 (2002).
Von Manteuffel, S. et al. 4E-Bp1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc. Natl Acad. Sci. USA 93, 4076–4080 (1996).
Brunn, G. J. et al. Three mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J. Biol. Chem. 272, 32547–32550 (1997).
Fadden, P. Jr. Identification of phosphorylation sites in the translational regulator, PHAS-I, that are controlled by insulin and rapamycin in rat adipocytes. J. Biol. Chem. 272, 10240–10247 (1997).
Herbert, P. T., Tee, A. R. & Proud, C. G. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J. Biol. Chem. 277, 11591–11596 (2002).
Di Como, C. J. & Arndt, K. T. Nutrients, via TOR proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 10, 1904–1916 (1996).
Murata, K., Wu, J. & Brautigan, D. L. B cell receptor-associated protein α4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc. Natl Acad. Sci. USA 148, 71–82 (1997).
Park, I.-H. et al. Regulation of ribosomal S6 kinase 2 by mammalian target of Rapamycin. J. Biol. Chem. 277, 31423–31429 (2002).
Seufferlein, T. & Rozengurt, E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer Res. 56, 3895–3897 (1996).
Brown EJ, Beal PA, Keith CT et al. Control of p70s6 kinase by kinase activity of FRAP in vivo. Nature 377, 441–446 (1995).
Burnett, P. E. et al. RAFT 1 phosphorylation of the translational regulators p70 s6 kinase and 4E-BP1. Proc. Natl Acad. Sci. USA 95, 1432–1437 (1998).
Dennis, P. B. et al. The principal rapamycin-sensitive p70s6k phosphorylation sites T229 and T389 are differentially regulated by rapamycin-insensitive kinase-kinases. Mol. Cell Biol. 16, 6242–6251 (1996).
Begum, N. & Ragolia, L. cAMP counter-regulates insulin-mediated protein phosphatase-2A inactivation in rat skeletal muscle cells. J. Biol. Chem. 271, 31166–31171 (1996).
Nourse, J. et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372, 570–573 (1994).
Grewe., M. et al. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 59, 3581–3587 (1999).
Kawamata, S. et al. The upregulation of p27Kip1 by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood 91, 561–569 (1998).
Hashemolhosseini, S. et al. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J. Biol. Chem. 273, 14424–14429 (1998).
Morice, W. G. et al. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J. Biol. Chem. 268, 22737–22745 (1993).
Luo, Y. et al. Rapamycin resistance tied to defective regulation of p27Kip1. Mol. Cell. Biol. 16, 6744–6751 (1996).
Rosenwald, I. B. et al. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J. Biol. Chem. 270, 21176–21180 (1995).
Mahajan, P. B. Modulation of transcription of rRNA genes by rapamycin. Int. J. Immunopharmacol. 16, 711–721 (1994).
Leicht, M. et al. Okadaic acid induces cellular hypertrophy in AKR-2B fibroblasts: involvment of the p70S6 kinase in the onset of protein and rRNA synthesis. Cell Growth Differ. 7, 1199–1209 (1996).
White, R. J. Regulation of RNA polymerases I and III by the retinoblastoma protein: a mechanism for growth control? Trends Biochem. Sci. 22, 77–80 (1997).
Rodriguez-Viciana, P. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994).
Feng, Z., Zhang, H., Levine, A. J. & Jin, S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl Acad. Sci. USA 102, 8204–8209 (2005).
Kanamori, Y. et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin. Cancer Res. 7, 892–895 (2001).
Uegaki, K. et al. PTEN-positive and phosphorylated-Akt-negative expression is a predictor of survival for patients with advanced endometrial carcinoma. Oncol. Rep. 14, 389–392 (2005).
Soliman, P. T. et al. mTOR inhibition as a potential treatment for progesterone-refractory endometrial hyperplasia. Proc. Am. Soc. Clin. Oncol. 23, 474s, A5080 (2005).
Choe, G. et al. Analysis of the phosphatidylinositol 3-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 63, 2742–2746 (2003).
Chakravarti, A. et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J. Clin. Oncol. 22, 1926–1933 (2004).
Chandrasekar, N. et al. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene 22, 392–400 (2003).
Frisk, T. et al. Silencing of the PTEN tumor-suppressor gene in anaplastic thyroid cancer. Genes Chromosomes Cancer 35, 74–80 (2002).
Virolle, T. et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nature Cell Biol. 3, 1124–1128 (2001).
Tell, G. et al. Control of phosphatase and tensin homolog (PTEN) gene expression in normal and neoplastic thyroid cells. Endocrinology 145, 4660–4666 (2004).
Lee, H. Y. et al. Evidence that phosphatidylinositol 3-Kinase-and mitogen-activated protein kinase kinase-4/c-Jun NH2-terminal kinase-dependent pathways cooperate to maintain lung cancer cell survival. J. Biol. Chem. 278, 23630–23638 (2003).
Marsit, C. J. et al. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 36, 768–776 (2005).
Ferraro, B., Bepler, G., Sharma, S., Cantor, A. & Haura, E. B. EGR1 predicts PTEN and survival in patients with non-small-cell lung cancer. J. Clin. Oncol. 23, 1921–1926 (2005).
Su, T. H. et al. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in cervical cancer. Gynecol. Oncol. 76, 193–199 (2000).
Harima, Y. et al. Mutation of the PTEN gene in advanced cervical cancer correlated with tumor progression and poor outcome after radiotherapy. Int. J. Oncol. 18, 493–497 (2001).
Cheung, T. H. et al. Epigenetic and genetic alternation of PTEN in cervical neoplasm. Gynecol. Oncol. 93, 621–627 (2004).
Tsutsui, S. et al. Reduced expression of PTEN protein and its prognostic implications in invasive ductal carcinoma of the breast. Oncology 68, 398–404 (2005).
Shoman, N. et al. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod. Pathol. 18, 250–259 (2005).
Saal, L. H. et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 65, 2554–2559 (2005).
Kirkegaard, T. et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J. Pathol. 207, 139–146 (2005).
Saito, M. et al. Allelic imbalance and mutations of the PTEN gene in ovarian cancer. Int. J. Cancer 85, 160–165 (2000).
Levine, D. A. et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin. Cancer Res. 11, 2875–2878 (2005).
Koksal, I. T. et al. The assessment of PTEN tumor suppressor gene in combination with Gleason scoring and serum PSA to evaluate progression of prostate carcinoma. Urol. Oncol. 22, 307–312 (2004).
Pfeil, K. et al. Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells. Prostate 58, 259–268 (2004).
Edwards, J., Krishna, N. S., Witton, C. J. & Bartlett, J. M. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin. Cancer Res. 9, 5271–5281 (2003).
Okami, K. et al. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumours. Cancer Res. 58, 509–511 (1998).
Kubo, Y., Urano, Y., Hida, Y. & Arase, S. Lack of somatic mutation in the PTEN gene in squamous cell carcinoma of human skin. J. Dermatol. Sci. 19, 199–201 (1999).
Aoki, K. et al. Cisplatin activates survival signals in UM-SCC-23 squamous cell carcinoma and these signal pathways are amplified in cisplatin-resistant squamous cell carcinoma. Oncol. Rep. 11, 375–379 (2004).
Khaleghpour, K., Li, Y., Banville, D., Yu, Z. & Shen, S. H. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis 25, 241–248 (2004).
Velho, S. et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur. J. Cancer 41, 1649–1654 (2005).
Agbunag, C. & Bar-Sagi, D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 64, 5659–5663 (2004).
Altomare, D. A. et al. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J. Cell Biochem. 88, 470–476 (2003).
Li, V. S. et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer 5, 29 (2005).
Oki, E. et al. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int. J. Cancer 117, 376–380 (2005).
Zhang, L., Yu, Q., He, J. & Zha, X. Study of the PTEN gene expression and FAK phosphorylation in human hepatocarcinoma tissues and cell lines. Mol. Cell Biochem. 262, 25–33 (2004).
Ma, D. Z. et al. Down-regulation of PTEN expression due to loss of promoter activity in human hepatocellular carcinoma cell lines. World J. Gastroenterol. 11, 4472–4477 (2005).
Vezina, C., Kudelski, A. & Sehgal, S. N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 28, 721–726 (1975).
Yatscoff, R. W., LeGatt, D. F. & Kneteman, N. M. Therapeutic monitoring of rapamycin: a new immunosuppressive drug. Ther. Drug Monit. 15, 478–482 (1993).
Davies, C. B. et al. Effect of a short course of rapamycin, cyclosporin A, and donor-specific transfusion on rat cardiac allograft survival. Transplantation 55, 1107–1112 (1993).
Kahan, B. D. Optimization of cyclosporine therapy. Transplant. Proc. 25, 5–9 (1993).
Trepanier, D. J. et al. Rapamycin: distribution, pharmacokinetics and therapeutic range investigations: an update. Clin. Biochem. 31, 345–351 (1998).
Kauffman, H. M., Cherikh, W. S., Cheng, Y., Hanto, D. W. & Kahan, B. D. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation 80, 883–889 (2005).
Gallo, R. et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation 99, 2164–2170 (1999).
Sousa, J. E. et al. Use of rapamycin-impregnated stents in coronary arteries. Transplant. Proc. 35, 165S–170S (2003).
Mohacsi, P. J. et al. Different inhibitory effects of immunosuppressive drugs on human and rat aortic smooth muscle and endothelial cell proliferation stimulated by platelet-derived growth factor or endothelial cell growth factor. J. Heart Lung Transplant. 16, 484–492 (1997).
Martin, D. E. & Hall, M. N. The expanding TOR signaling network. Curr. Opin. Cell. Biol. 17, 158–166 (2005).
Ravikumar, B. et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nature Genet. 36, 585–595 (2004).
Li, X., Alafuzoff, I., Soininen, H., Winblad, B. & Pei, J. J. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 272, 4211–4220 (2005).
Nardacci, R. et al. Characterization of cell death pathways in human immunodeficiency virus-associated encephalitis. Am. J. Pathol. 167, 695–704 (2005).
Castedo, M. et al. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 21, 4070–4080 (2002).
Busca, R. et al. Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J. Biol. Chem. 271, 31824–31830 (1996).
Grewe, M. et al. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP–p70s6K pathway in human pancreatic cancer cells. Cancer Res. 59, 3581–3587 (1999).
Hosoi, H. et al. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 59, 886–894 (1999).
Shi, Y. et al. Rapamycin enhances apoptosis and increases sensitivity to cisplatin in vitro. Cancer Res. 55, 1982–1988 (1995).
Calastretti, A. et al., Damaged microtubules can inactivate BCL-2 by means of the mTOR kinase. Oncogene 20, 6172–6180 (2001).
Balcarcel, R. R. & Stephanopoulos, G. Rapamycin reduces hybridoma cell death and enhances monoclonal antibody production. Biotechnol. Bioeng. 76, 1–10 (2001).
Humar, R. et al. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 16, 771–780 (2002). A report on the role of hypoxia and consequences in activation of cell signalling in endothelial cells, proliferation and tumour angiogenesis.
Castedo, T. et al. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 21, 4070–4080 (2002).
Decaudin, D. et al. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 57, 62–67 (1997).
Zangemeister-Wittke, U. et al. A novel bispecific antisense oligonucleotide inhibiting both BCL-2 and BCL-xL expression efficiently induces apoptosis in tumor cells. Clin. Cancer Res. 6, 2547–2555 (2000).
Shinjyo, T. et al. Downregulation of bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol. Cell Biol. 21, 854–864 (2001).
Shinoura, N. et al. Expression level of bcl-2 determines anti- or proapoptotic function. Cancer Res. 59, 4119–4128 (1999).
Aguirre, D. et al. Bcl-2 and CCND1/CDK4 expression levels predict the cellular effects of mTOR inhibitors in human ovarian carcinoma. Apoptosis 9, 797–805 (2004). This paper underlines the role of BCL2 expression as a molecular factor of resistance to mTOR inhibitors in human cancer cells.
Boya, P. et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell Biol. 25, 1025–1040 (2005).
Kroemer, G. & Jaattela, M. Lysosomes and autophagy in cell death control. Nature Rev. Cancer. 11, 886–897 (2005).
Hamada, K. et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Gene Dev. 19, 2054–2065 (2005).
Guba, M. et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Med. 8, 128–135 (2002). Describes the modulation of cross-talk between PI3K/AKT and VEGF signalling pathways by rapamycin.
Humar, R., Kiefer, F. N., Berns, H., Resink, T. J. & Battegay, E. J. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 16, 771–780 (2002).
Arsham, A. M., Plas, D. R., Thompson, C. B. & Simon, M. C. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1α nor sufficient for HIF-1-dependent target gene transcription. J. Biol. Chem. 277, 15162–15170 (2002).
Trisciuoglio, D., Iervolino, A., Zupi, G. & Del Bufalo, D. Involvment of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol. Biol. Cell 16, 4153–4162 (2005).
Costa, L. F., Balcells, M., Edelman, E. R., Nadler, L. M. & Cardoso, A. A. Pro-angiogenic stimultation of bone marrow endothelium engages mTOR, and is inhibited by simultaneous blockade of mTOR and NF-κB. Blood 107, 285–292 (2006).
Thomas, G. V. et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nature Med. 12, 122–127 (2006).
Raymond, E. et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J. Clin. Oncol. 16, 2336–2347 (2004). This paper reports the first-in-man experience (phase I trial) using CCI-779 (temsirolimus, a potent mTOR inhibitor) in patients with advanced cancers, describing the first evidence of antitumour activity in patients with renal cell carcinoma.
Hidalgo, M. et al. CCI-779, a rapamycin analog and multifaceted inhibitor of signal transduction: a phase I study. Proc. Am. Soc. Clin. Oncol. 19, 187a, A726 (2000).
O'Donnell, A. et al. A phase I study of the oral mTOR inhibitor RAD001 as monotherapy to identify the optimal biologically effective dose using toxicity, pharmacokinetic (PK) and pharmacodynamic (PD) endpoints in patients with solid tumors. Proc. Am. Soc. Clin. Oncol. 22, 200, A803 (2003).
Mita, M. AP23573, an mTOR inhibitor, administered IV daily × 5 every other week in patients with refractory or advanced malignancies — a phase I, pharmacokinetic (PK), and pharmacodynamic (PD) study. Proc. 16th Symp. Mol. Targets Cancer Thera. Geneva, Switzerland, October, 122 A409 (2004).
Oza, A. M. et al. A phase II study or tensirolimus (CCI-779) in patients with metastatic and/or locally recurrent endometrial cancer. Proc. 17th Symp. Mol. Targets Cancer Thera. Philadelphia, USA, November, 197 AB269 (2005).
Galanis, E. et al. Phase II trial of tensirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol. 23, 5294–5304 (2005).
Chang, S. M. et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest. New Drugs 23, 357–361 (2005).
Atkins, M. B. et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 22, 909–198 (2004).
Hudes, G. et al. A phase 3, randomised, 3-arm study of temsirolimus (TEMSR) or interferon-α (IFN) or the combination of TEMSR + IFR in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma. J. Clin. Oncol. 24, Abs. LBA 4, 18S (2006).
Amato, R. J., Misellati, A., Khan, M. & Chiang, S. A phase II trial of RAD001 in patients wih metastatic renal cell carcinoma. J. Clin. Oncol. 24, A4530, 18S (2006).
Chan, S. et al. Phase II study of tensirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 23, 5314–5322 (2005).
Witzig, T. E. et al. Phase II trial of single-agent tensirolimus (CCI-779) for relapsed mantle cell lymphoma. J. Clin. Oncol. 23, 5347–5356 (2005). A report of the remarkable antitumour activity of CCI-779 (temsirolimus) in mantle cell lymphoma, a disease driven by cyclin D1 overexpression that can be controlled using mTOR inhibitors.
Ansell, S. M. et al. Anti-tumor activity of mTOR inhibitor temsirolimus for relapse mantle cell lymphoma: a phase II trial in the North Central Cancer Treatment Group. J. Clin. Oncol. 24 18S, A7532 (2006).
Yao, J. C. et al. Phase II study of RAD001 (everolimus) and depot octreotide (sandostatine LAR) in patients with advanced low grade neuroendocrine carcinoma. J. Clin. Oncol. 24, 18S, A4042 (2006).
Duran, I. et al. A phase II trial of temsirolimus in metastatic neuroendocrine carcinoma. Proc. Am. Soc. Clin. Oncol. 24, 824s, A146 (2005).
von Oosterom, A. et al. A phase I/II trial of the oral mTOR-inhibitor everolimus (E) and imatinib mesylate (IM) in patients (pts) with gastrointestinal stromal tumor (GIST) refractory to IM: study update. Proc. Am. Soc. Clin. Oncol. 24, 824s, A9033 (2005).
Chawla, S. P. et al. A phase II trial of AP23573, a novel mTOR inhibitor, in patients (pts) with advanced soft tissue or bone sarcoma. Proc. 17th Symp. Mol. Targets Cancer Thera., Philadelphia, USA, November, 268 AC272 (2005).
Boulay, A. et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 64, 252–261 (2004). This research paper reports that S6K1 phosphorylation can be used as a molecular marker for mTOR inhibitors in peripheral blood mononuclear cells, paving the way for the use of S6K1 in translational clinical research.
Duran, I. et al. Pharmacodynamic evaluations of paired tumor biopsies in advanced neuroendocrine carcinomas (NECs) treated with the mTOR inhibitor tensirolimus. Proc. 17th Symp. Mol. Targets Cancer Thera., Philadelphia, USA, November, 230 AC128 (2005).
Paralba, J. M. et al. Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clin. Cancer Res. 9, 2887–2892 (2003).
Tabernero, J. et al. A phase I study with tumor molecular pharmacodynamic (MPD) evaluation of dose and schedule of the oral mTOR inhibitor everolimus (RAD001) in patients (pts) with advanced solid tumors. Proc. Am. Soc. Clin. Oncol. 24, 193s, A3007 (2005).
Reardon, D. A. et al. A phase I trial of AP23573, a novel mTOR inhibitor, in patients (pts) with recurrent malignant glioma. Proc. 17th Symp. Mol. Targets Cancer Thera., Philadelphia, USA, November, 105 AC195 (2005).
Rivera, V. et al. Pharmacodynamic evaluation of the mTOR inhibitor AP23573 in phase I clinical trials. Proc. 16th Symp. Mol. Targets Cancer Thera. Geneva, Switzerland, October, 123 A411 (2004).
Rivera, V. M. et al. Pharmacodynamic study of skin biopsy specimens in patients (pts) with refractory or advanced malignancies following administration of AP23573, an mTOR inhibitor. Proc. Am. Soc. Clin. Oncol. 24, 200s, A3033 (2005).
Sankhala, K. K. et al. Early response evaluation of therapy with AP23573 (an mTOR inhibitor) in sarcoma using [18F]2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) scan. Proc. Am. Soc. Clin. Oncol. 24, 823s, A9028 (2005).
Cho, D. C. et al. Low expression of surrogates for mTOR pathway activation predicts resistance to CCI-779 in patients with advanced renal cell carcinoma (RCC). Proc. 17th Symp. Mol. Targets Cancer Thera. Philadelphia, USA, November, 233 C137 (2005).
Diehl, K. M. et al. In-vitro studies of rapamycin treatment of osteosarcoma cell lines. Proc. 17th Symp. Mol. Targets Cancer Thera., Philadelphia, USA, November, 210 AC53 (2005).
Shah, O. J., Wang, Z. & Hunter, T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14, 1650–1656 (2004).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Cheng, J. Q. et al. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 24, 7482–7492 (2005).
Guertin, D. A. & Sabatini, D. M. An expanding role for mTOR in cancer. Trends Mol. Med. 11, 353–361 (2005).
Takeuchi, H. et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 65, 3336–3346 (2005).
Sun, S. Y. et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 65, 7052–7058 (2005).
Oki, E. et al. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int. J. Cancer 117, 376–380 (2005).
Aoki, K. et al. Cisplatin activates survival signals in UM-SCC-23 squamous cell carcinoma and these signal pathways are amplified in cisplatin-resistant squamous cell carcinoma. Onc. Rep. 11, 375–379 (2004).
Beuvink, I. et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 120, 747–759 (2005).
Dong, J. et al. Role of glycogen synthase kinase 3β in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Res. 65, 1961–1972 (2005).
Nagata, Y. et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6, 117–127 (2004). An exploration of the functional role of PTEN for sensitivity to trastuzumab, a HER2 inhibitor, in breast cancer cells.
Kokubo, Y. et al. Reduction of PTEN protein and lss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA). Br. J. Cancer 92, 1711–1719 (2005).
Milton, D. T. et al. PhaseI/II trial of gefitinib and RAD001 (everolimus) in patients (pts) with advanced non-small cell lung cancer (NSCLC). Proc. Am. Soc. Clin. Oncol. 24, 646s, A7104 (2005).
deGraffenried, L. A. et al. Inhibition of mTOR activity restores tamoxifene response in breast cancer cells with aberant Akt activity. Clin. Cancer Res. 10, 8059–8067 (2004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Receptor tyrosine kinases
-
(RTKs). A family of transmembrane receptors that are physiologically activated by the extracellular binding of growth factor(s) and which initiate intracellular signalling, ultimately leading to many cellular responses such as proliferation. Abnormalities of these receptors are often reported in human malignancies.
- Translational research
-
Research aimed at using biological tools for clinical applications.
- Glioblastoma
-
High-grade brain malignancy arising from astrocytes with abnormal cellular proliferation and increased tumour angiogenesis. This cancer is usually refractory to chemotherapy and has a very poor prognosis.
- Mucositis
-
Inflammation and/or ulceration of mouth mucosa.
- Thrombocytopaenia
-
Low level of platelets in circulating blood.
- Objective response
-
Shrinkage of at least 50% of malignant target lesions (according to World Health Organization criteria) after administration of anticancer treatment as compared with baseline measurement.
- Sarcoma
-
Malignant tumour arising from the bone (osteosarcoma) or the soft tissues with high risk of lung metastasis. Patients with soft tissue sarcomas are frequently poor responders to classical chemotherapy.
Rights and permissions
About this article
Cite this article
Faivre, S., Kroemer, G. & Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 5, 671–688 (2006). https://doi.org/10.1038/nrd2062
Issue Date:
DOI: https://doi.org/10.1038/nrd2062
This article is cited by
-
Conjugation of VEGFR1/R2-targeting peptide with gold nanoparticles to enhance antiangiogenic and antitumoral activity
Journal of Nanobiotechnology (2022)
-
Perfect match: mTOR inhibitors and tuberous sclerosis complex
Orphanet Journal of Rare Diseases (2022)
-
Recent advances and limitations of mTOR inhibitors in the treatment of cancer
Cancer Cell International (2022)
-
Everolimus versus sirolimus for angiomyolipoma associated with tuberous sclerosis complex: a multi-institutional retrospective study in China
Orphanet Journal of Rare Diseases (2021)
-
A Systems Biology Roadmap to Decode mTOR Control System in Cancer
Interdisciplinary Sciences: Computational Life Sciences (2020)