Key Points
-
Recent research progress into the molecular pathogenesis of Alzheimer's disease has been translated into several promising drug candidates that have the potential to produce disease-modifying effects.
-
Biomarkers that reflect the central pathogenic processes in Alzheimer's disease have been developed and validated in numerous studies. These include cerebrospinal fluid (CSF) assays for tau and amyloid-β isoforms; magnetic resonance imaging (MRI) measurements of brain atrophy; and positron emission tomography (PET) techniques for brain metabolism and amyloid-β deposition.
-
Academic institutions, the pharmaceutical industry and regulatory organizations all agree that biomarkers have an important role in the drug development process.
-
Biomarkers have several potential uses in clinical trials. These include their use as diagnostic aids to enrich the patient sample with cases of Alzheimer's disease; as tools to identify and monitor the biochemical effect of the drug candidate; and as safety markers to detect potential side effects of the drug.
-
Evidence obtained from biomarker studies showing that a drug candidate affects the central disease processes in Alzheimer's disease will, together with a beneficial effect on cognition, be essential for the drug to be labelled as disease-modifying.
-
A catch-22-like situation exists in validating Alzheimer's disease biomarkers for use in drug development. Biomarker validation depends on effective drugs that target Alzheimer's disease pathogenesis, which are not currently available. At the same time, evidence from biomarker studies is needed for a new drug to be labelled as disease-modifying.
-
There are numerous ongoing clinical trials investigating disease-modifying drug candidates, which include biomarkers as end points. These trials will provide information on whether biomarkers will be valuable tools as surrogate end points to predict the clinical outcome and as the basis for a disease-modifying claim of the drug.
-
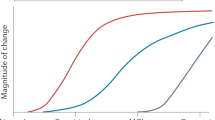
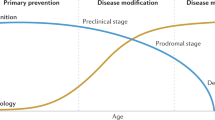
If disease-modifying drugs are approved for the treatment of Alzheimer's disease, biomarkers will facilitate the diagnosis of Alzheimer's disease very early on in the course of the disease before neurodegeneration is too severe and widespread.
Abstract
Advances in therapeutic strategies for Alzheimer's disease that lead to even small delays in onset and progression of the condition would significantly reduce the global burden of the disease. To effectively test compounds for Alzheimer's disease and bring therapy to individuals as early as possible there is an urgent need for collaboration between academic institutions, industry and regulatory organizations for the establishment of standards and networks for the identification and qualification of biological marker candidates. Biomarkers are needed to monitor drug safety, to identify individuals who are most likely to respond to specific treatments, to stratify presymptomatic patients and to quantify the benefits of treatments. Biomarkers that achieve these characteristics should enable objective business decisions in portfolio management and facilitate regulatory approval of new therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blennow, K., de Leon, M. J. & Zetterberg, H. Alzheimer's disease. Lancet 368, 387–403 (2006).
Klafki, H. W., Staufenbiel, M., Kornhuber, J. & Wiltfang, J. Therapeutic approaches to Alzheimer's disease. Brain 129, 2840–2855, (2006).
Garcia-Alloza, M. et al. Existing plaques and neuritic abnormalities in APP:PS1 mice are not affected by administration of the gamma-secretase inhibitor LY-411575. Mol. Neurodegener. 4, 19 (2009).
Das, P., Murphy, M. P., Younkin, L. H., Younkin, S. G. & Golde, T. E. Reduced effectiveness of Aβ1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol. Aging 22, 721–727 (2001).
Levites, Y. et al. Anti-Aβ42- and anti-Aβ40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J. Clin. Invest. 116, 193–201 (2006).
Brookmeyer, R., Johnson, E., Ziegler-Graham, K., Arrighi, H. M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 3, 186–191 (2007).
Siemers, E. R. How can we recognize “disease modification” effects? J. Nutr. Health Aging 13, 341–343 (2009).
Frank, R. & Hargreaves, R. Clinical biomarkers in drug discovery and development. Nature Rev. Drug Discov. 2, 566–580, (2003).
Frank, R. A. et al. Biological markers for therapeutic trials in Alzheimer's disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer's disease. Neurobiol. Aging 24, 521–536, (2003).
Orgogozo, J. M. et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 61, 46–54 (2003).
Blennow, K. & Hampel, H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2, 605–613 (2003).
Teipel, S. J., Meindl, T., Grinberg, L., Heinsen, H. & Hampel, H. Novel MRI techniques in the assessment of dementia. Eur. J. Nucl. Med. Mol. Imaging 35 (Suppl. 1), 58–69 (2008).
Hampel, H. et al. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 4, 38–48 (2008).
Whiting, P., Rutjes, A. W., Reitsma, J. B., Bossuyt, P. M. & Kleijnen, J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 3, 25 (2003).
Nagy, Z. et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia 7, 76–81 (1996).
Logothetis, N. K. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1003–1037 (2002).
Reivich, M. et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ. Res. 44, 127–137 (1979).
Lockhart, A. et al. PIB is a non-specific imaging marker of amyloid-β (Aβ) peptide-related cerebral amyloidosis. Brain 130, 2607–2615 (2007).
Allegri, R. F., Glaser, F. B., Taragano, F. E. & Buschke, H. Mild cognitive impairment: believe it or not? Int. Rev. Psychiatry 20, 357–363 (2008).
Fennema-Notestine, C. et al. Structural neuroimaging in the detection and prognosis of pre-clinical and early AD. Behav. Neurol. 21, 3–12 (2009).
Giedd, J. N. et al. Reliability of cerebral measures in repeated examinations with magnetic resonance imaging. Psychiatry Res. 61, 113–119 (1995).
Ewers, M. et al. Multicenter assessment of reliability of cranial MRI. Neurobiol. Aging 27, 1051–1059 (2006).
Bokde, A. L. et al. Decreased activation along the dorsal visual pathway after a 3-month treatment with galantamine in mild Alzheimer disease: a functional magnetic resonance imaging study. J. Clin. Psychopharmacol. 29, 147–156 (2009).
Moffett, J. R., Ross, B., Arun, P., Madhavarao, C. N. & Namboodiri, A. M. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 81, 89–131 (2007).
Kantarci, K. 1H magnetic resonance spectroscopy in dementia. Br. J. Radiol. 80, S146–S152 (2007).
Alexander, G. E., Chen, K., Pietrini, P., Rapoport, S. I. & Reiman, E. M. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer's disease treatment studies. Am. J. Psychiatry 159, 738–745 (2002).
Hirono, N., Hashimoto, M., Ishii, K., Kazui, H. & Mori, E. One-year change in cerebral glucose metabolism in patients with Alzheimer's disease. J. Neuropsychiatry Clin. Neurosci. 16, 488–492 (2004).
Heiss, W. D., Kessler, J., Mielke, R., Szelies, B. & Herholz, K. Long-term effects of phosphatidylserine, pyritinol, and cognitive training in Alzheimer's disease. A neuropsychological, EEG, and PET investigation. Dementia 5, 88–98 (1994).
Mega, M. S. et al. Metabolic patterns associated with the clinical response to galantamine therapy: a fludeoxyglucose F18 positron emission tomographic study. Arch. Neurol. 62, 721–728 (2005).
Stefanova, E. et al. Longitudinal PET evaluation of cerebral glucose metabolism in rivastigmine treated patients with mild Alzheimer's disease. J. Neural Transm. 113, 205–218 (2006).
Kadir, A. et al. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer's disease. Ann. Neurol. 63, 621–631 (2008).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 55, 306–319 (2004).
Jack, C. R. Jr et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 131, 665–680 (2008).
Maeda, J. et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer's disease enabled by positron emission tomography. J. Neurosci. 27, 10957–10968 (2007).
Villemagne, V. L. et al. Aβ deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia 46, 1688–1697 (2008).
Forsberg, A. et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol. Aging 29, 1456–1465, (2008).
Rowe, C. C. et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 7, 129–135 (2008).
Bobinski, M. et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience 95, 721–725 (2000).
Vellas, B. et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr. Alzheimer Res. 6, 144–151 (2009).
Saumier, D. et al. Lessons learned in the use of volumetric MRI in therapeutic trials in Alzheimer's disease: the ALZHEMED (Tramiprosate) experience. J. Nutr. Health Aging 13, 370–372 (2009).
Harold, D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nature Genet. 41, 1088–1093 (2009).
Lambert, J. C. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature Genet. 41, 1094–1099 (2009).
Klunk, W. E. et al. Imaging the pathology of Alzheimer's disease: amyloid-imaging with positron emission tomography. Neuroimaging Clin. N. Am. 13, 781–789 (2003).
Chen, K. et al. Correlations between apolipoprotein E ɛ4 gene dose and whole brain atrophy rates. Am. J. Psychiatry 164, 916–921 (2007).
Scahill, R. I., Schott, J. M., Stevens, J. M., Rossor, M. N. & Fox, N. C. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc. Natl Acad. Sci. USA 99, 4703–4707 (2002).
Scheuner, D. et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nature Med. 2, 864–870 (1996).
Myers, A. J. et al. A survey of genetic human cortical gene expression. Nature Genet. 39, 1494–1499 (2007).
Kauwe, J. S. et al. Alzheimer's disease risk variants show association with cerebrospinal fluid amyloid β. Neurogenetics 10, 13–17 (2009).
Kauwe, J. S. et al. Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-β deposition. Proc. Natl Acad. Sci. USA 105, 8050–8054 (2008).
Melzer, D. et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 4, e1000072 (2008).
Makeeva, O., Stepanov, V., Puzyrev, V., Goldstein, D. B. & Grossman, I. Global pharmacogenetics: genetic substructure of Eurasian populations and its effect on variants of drug-metabolizing enzymes. Pharmacogenomics 9, 847–868 (2008).
Farrer, L. A. et al. Statement on use of apolipoprotein E testing for Alzheimer disease. American College of Medical Genetics/American Society of Human Genetics Working Group on ApoE and Alzheimer disease. JAMA 274, 1627–1629 (1995).
Green, R. C. et al. Disclosure of APOE genotype for risk of Alzheimer's disease. N. Engl. J. Med. 361, 245–254 (2009).
Herukka, S. K. et al. CSF Aβ42, Tau and phosphorylated Tau, APOE ɛ4 allele and MCI type in progressive MCI. Neurobiol. Aging 28, 507–514 (2007).
Ray, S. et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nature Med. 13, 1359–1362 (2007).
Irizarry, M. C. Biomarkers of Alzheimer disease in plasma. NeuroRx 1, 226–234 (2004).
Vanderstichele, H. et al. Standardization of measurement of beta-amyloid(1–42) in cerebrospinal fluid and plasma. Amyloid 7, 245–258 (2000).
Peskind, E. R. et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis. Assoc. Disord. 19, 220–225 (2005).
Blennow, K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx 1, 213–225 (2004).
Strozyk, D., Blennow, K., White, L. R. & Launer, L. J. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60, 652–656 (2003).
Fagan, A. M. et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann. Neurol. 59, 512–519 (2006).
Buerger, K. et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain 129, 3035–3041 (2006).
Shaw, L. M. et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413, (2009).
Visser, P. J. et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 8, 619–627, (2009).
Mattsson, N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393, (2009).
Andreasson, U., Portelius, E., Andersson, M. E., Blennow, K. & Zetterberg, H. Aspects of β-amyloid as a biomarker for Alzheimer's disease. Biomarkers Med. 1, 59–78 (2007).
de Jong, D., Kremer, B. P., Olde Rikkert, M. G. & Verbeek, M. M. Current state and future directions of neurochemical biomarkers for Alzheimer's disease. Clin. Chem. Lab. Med. 45, 1421–1434 (2007).
Zhong, Z. et al. Levels of β-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch. Gen. Psychiatry 64, 718–726 (2007).
Zetterberg, H. et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch. Neurol. 65, 1102–1107 (2008).
Davidsson, P. et al. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer's disease. Neurosci. Lett. 300, 157–160 (2001).
von der Kammer, H. et al. Muscarinic acetylcholine receptors activate expression of the EGR gene family of transcription factors. J. Biol. Chem. 273, 14538–14544 (1998).
Blennow, K. et al. Longitudinal stability of CSF biomarkers in Alzheimer's disease. Neurosci. Lett. 419, 18–22 (2007).
Zetterberg, H. et al. Intra-individual stability of CSF biomarkers for Alzheimer's disease over two years. J. Alzheimers Dis. 12, 255–260 (2007).
Lanz, T. A., Hosley, J. D., Adams, W. J. & Merchant, K. M. Studies of Aβ pharmacodynamics in the brain, cerebrospinal fluid, and plasma in young (plaque-free) Tg2576 mice using the γ-secretase inhibitor N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b, d]azepin-7-yl]-L-alaninamide (LY-411575). J. Pharmacol. Exp. Ther. 309, 49–55 (2004).
Anderson, J. J. et al. Reductions in β-amyloid concentrations in vivo by the γ-secretase inhibitors BMS-289948 and BMS-299897. Biochem. Pharmacol. 69, 689–698 (2005).
Fleisher, A. S. et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch. Neurol. 65, 1031–1038 (2008).
Sankaranarayanan, S. et al. First demonstration of cerebrospinal fluid and plasma Aβ lowering with oral administration of a β-site amyloid precursor protein-cleaving enzyme 1 inhibitor in nonhuman primates. J. Pharmacol. Exp. Ther. 328, 131–140 (2009).
Adlard, P. A. et al. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Aβ. Neuron 59, 43–55 (2008).
Lannfelt, L. et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 7, 779–786 (2008).
Hesse, C. et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 297, 187–190 (2001).
Zetterberg, H. et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277–1280 (2006).
Gilman, S. et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64, 1553–1562 (2005).
Degerman Gunnarsson, M., Kilander, L., Basun, H. & Lannfelt, L. Reduction of phosphorylated tau during memantine treatment of Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 24, 247–252 (2007).
Slamon, D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712 (1989).
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 (2007).
Carrillo, M. C. et al. Early risk assessment for Alzheimer's disease. Alzheimers Dement. 5, 182–196 (2009).
Coats, M. & Morris, J. C. Antecedent biomarkers of Alzheimer's disease: the adult children study. J. Geriatr. Psychiatry Neurol. 18, 242–244 (2005).
Mintun, M. A. et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452 (2006).
Mani, R. B. The evaluation of disease modifying therapies in Alzheimer's disease: a regulatory viewpoint. Stat. Med. 23, 305–314 (2004).
Pepe, M. S. et al. Phases of biomarker development for early detection of cancer. J. Natl Cancer Inst. 93, 1054–1061 (2001).
Leber, P. Slowing the progression of Alzheimer disease: methodologic issues. Alzheimer Dis. Assoc. Disord. 11 (Suppl. 5), 10–21; discussion 37–19 (1997).
Woodcock, J. & Woosley, R. The FDA critical path initiative and its influence on new drug development. Annu. Rev. Med. 59, 1–12 (2008).
Katz, R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 1, 189–195 (2004).
Temple, R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 282, 790–795 (1999).
Baker, S. G. & Kramer, B. S. A perfect correlate does not a surrogate make. BMC Med. Res. Methodol. 3, 16 (2003).
Fleming, T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).
Fox, N. C. et al. Effects of Aβeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64, 1563–1572 (2005).
Jack, C. R. Jr et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27, 685–691 (2008).
Mueller, S. G. et al. Ways toward an early diagnosis in Alzheimer's disease: The Alzheimer's Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 1, 55–66 (2005).
Williams, S. A., Slavin, D. E., Wagner, J. A. & Webster, C. J. A cost-effectiveness approach to the qualification and acceptance of biomarkers. Nature Rev. Drug Discov. 5, 897–902 (2006).
Gutman, S. & Kessler, L. G. The US Food and Drug Administration perspective on cancer biomarker development. Nature Rev. Cancer 6, 565–571 (2006).
Grundman, M. & Black, R. O3-04-05: clinical trials of bapineuzumab, a β-amyloid-targeted immunotherapy in patients with mild to moderate Alzheimer's disease. Alzheimers Dement. 4, T166 (2008).
Sanhai, W. R., Spiegel, J. & Ferrari, M. A critical path approach to advance nanoengineered medical products. Drug Discov. Today Technol. 4, 35–41 (2007).
Ridha, B. H. et al. Application of automated medial temporal lobe atrophy scale to Alzheimer disease. Arch. Neurol. 64, 849–854 (2007).
Byrum, C. E. et al. Accuracy and reproducibility of brain and tissue volumes using a magnetic resonance segmentation method. Psychiatry Res. 67, 215–234 (1996).
Jack, C. R. Jr et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 49, 786–794 (1997).
Wang, P. N., Lirng, J. F., Lin, K. N., Chang, F. C. & Liu, H. C. Prediction of Alzheimer's disease in mild cognitive impairment: a prospective study in Taiwan. Neurobiol. Aging 27, 1797–1806, (2006).
Fox, N. C., Cousens, S., Scahill, R., Harvey, R. J. & Rossor, M. N. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch. Neurol. 57, 339–344 (2000).
Loubinoux, I. et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. J. Cereb. Blood Flow Metab. 21, 592–607 (2001).
Kircher, T. T., Erb, M., Grodd, W. & Leube, D. T. Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am. J. Geriatr. Psychiatry 13, 1006–1013 (2005).
Bokde, A. L. W. et al. Decreased activation along the dorsal visual pathway after a 3-month treatment with galantamine in mild Alzheimer disease. J. Clin. Psychopharmacol. 29, 147–156 (2009).
Goekoop, R., Scheltens, P., Barkhof, F. & Rombouts, S. A. Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation — a pharmacological fMRI study. Brain 129, 141–157 (2006).
Chao, L. L. et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology 64, 282–289 (2005).
Jessen, F. et al. Treatment monitoring and response prediction with proton MR spectroscopy in AD. Neurology 67, 528–530 (2006).
Herholz, K. et al. Comparability of FDG PET studies in probable Alzheimer's disease. J. Nucl. Med. 34, 1460–1466 (1993).
Edison, P. et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68, 501–508 (2007).
Heiss, W. D. et al. Effect of piracetam on cerebral glucose metabolism in Alzheimer's disease as measured by positron emission tomography. J. Cereb. Blood Flow Metab. 8, 613–617 (1988).
Teipel, S. J. et al. Effects of donepezil on cortical metabolic response to activation during 18FDG-PET in Alzheimer's disease: a double-blind cross-over trial. Psychopharmacology (Berl.) 187, 86–94 (2006).
Engler, H. et al. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain 129, 2856–2866 (2006).
Bohnen, N. I. et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 76, 315–319 (2005).
Andreasen, N. et al. Cerebrospinal fluid β-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch. Neurol. 56, 673–680 (1999).
Olsson, A. et al. Simultaneous measurement of β-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin. Chem. 51, 336–345 (2005).
Fukuyama, R. et al. Age-dependent change in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer's disease patients. Eur. Neurol. 43, 155–160 (2000).
Kanai, M. et al. Longitudinal study of cerebrospinal fluid levels of tau, Aβ1–40, and Aβeta1–42(43) in Alzheimer's disease: a study in Japan. Ann. Neurol. 44, 17–26 (1998).
Lewczuk, P. et al. Neurochemical diagnosis of Alzheimer's dementia by CSF Aβ42, Aβ42/Aβ40 ratio and total tau. Neurobiol. Aging 25, 273–281 (2004).
Hansson, O. et al. Prediction of Alzheimer's disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 23, 316–320 (2007).
Shoji, M. et al. Combination assay of CSF tau, Aβ1–40 and Aβ1–42(43) as a biochemical marker of Alzheimer's disease. J. Neurol. Sci. 158, 134–140 (1998).
de Leon, M. J. et al. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci. Lett. 333, 183–186 (2002).
de Leon, M. J. et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol. Aging 27, 394–401 (2006).
Olsson, A. et al. Measurement of α- and β-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp. Neurol. 183, 74–80 (2003).
Verheijen, J. H. et al. Detection of a soluble form of BACE-1 in human cerebrospinal fluid by a sensitive activity assay. Clin. Chem. 52, 1168–1174 (2006).
Holsinger, R. M., Lee, J. S., Boyd, A., Masters, C. L. & Collins, S. J. CSF BACE1 activity is increased in CJD and Alzheimer disease versus [corrected] other dementias. Neurology 67, 710–712 (2006).
Holsinger, R. M., McLean, C. A., Collins, S. J., Masters, C. L. & Evin, G. Increased beta-secretase activity in cerebrospinal fluid of Alzheimer's disease subjects. Ann. Neurol. 55, 898–899 (2004).
Georganopoulou, D. G. et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc. Natl Acad. Sci. USA 102, 2273–2276 (2005).
Vanderstichele, H. et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU(181P) assay for discrimination between Alzheimer's disease and dementia with Lewy bodies. Clin. Chem. Lab. Med. 44, 1472–1480 (2006).
Vanmechelen, E. et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci. Lett. 285, 49–52, (2000).
Kohnken, R. et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer's disease patients. Neurosci. Lett. 287, 187–190 (2000).
Hampel, H. et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch. Gen. Psychiatry 61, 95–102 (2004).
Acknowledgements
The authors would like to thank K. Duggan for technical assistance. H.H. acknowledges support by the Science Foundation Ireland Investigator Neuroimaging Grant Programme (08/IN.1/B1846).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Harald Hampel has participated on the Advisory Board of BRAHMS, Henningsdorf, Germany, and has received research support for his institutions from the same company.
Richard Frank is an employee of GE Healthcare.
John Hardy is a consultant for Eisai and MerckSerono.
Kaj Blennow has participated on the Advisory Board for Innogenetics NV, Ghent, Belgium, and has received research support to his institution from the same company.
Karl Broich, Stefan J. Teipel, Russel G. Katz, Karl Herholz, Arun L.W. Bokde, Frank Jessen, Yvonne C. Hoessler, Wendy R. Sanhai, Henrik Zetterberg and Janet Woodcock declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Imaging biomarkers for Alzheimer's disease (PDF 565 kb)
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Alzheimer's Association Research Roundtable
Improved Predictivity of Efficacy Evaluation — Brain Disorders
Improved Predictivity of Efficacy Evaluation — Brain Disorders
Glossary
- Amyloid-β
-
(Aβ). An aggregation-prone peptide derived from the amyloid precursor protein. The 42 amino acid isoform of the peptide is the main component of plaques in Alzeimer's disease.
- Tau protein
-
A microtubule-associated protein located in the neuronal axons. Hyperphosphorylated tau is the main component of neurofibrillary tangles in Alzheimer's disease.
- DSM-IV
-
(Diagnostic and Statistical Manual of Mental Disorders, Fourth edition). This manual outlines diagnostic criteria for psychiatric disorders and is published by the American Psychiatric Association.
- ICD-10
-
(International Statistical Classification of Diseases, Tenth edition). This book outlines diagnostic criteria for human diseases and is published by the World Health Organization.
- NINCDS-ADRDA
-
(The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association). This outlines criteria for diagnosing Alzheimer's disease.
- Primary end point
-
A primary end point is defined as the single main question to be answered in a given clinical trial.
- Biomarker
-
An objective measure of a biological or pathogenic process that can be used to evaluate disease risk or prognosis. It can be used to guide clinical diagnosis or to monitor therapeutic interventions.
- Surrogate end point
-
A substitute for a clinical end point in a clinical trial.
- Secondary end point
-
An end point in a trial that provides additional characterization of treatment effect, but is not sufficient by itself to fully characterize the benefit or to support a claim for a treatment effect.
- Longitudinal study
-
A research study with repeated observations of the same patients over long periods of time.
- Phantom test
-
A plastic cylinder with standardized measures and density used for calibration of magnetic resonance imaging devices.
- Transformation map
-
A spatially extended vector field that describes the spatial warps that are needed to align a three-dimensional image of the brain into a common standard space.
- Classical plaque
-
A dense aggregation of amyloid-β protein with classical amyloid characteristics, which is surrounded by swollen neuritis and reactive glial cells.
- Diffuse plaque
-
An aggregation of amyloid-β protein that can only be detected using immunohistochemistry.
- AN1792 vaccination trial
-
The first clinical trial on active amyloid-β immunotherapy in Alzheimer's disease.
- Huntington's protocol
-
Individuals who have family members with Huntington's disease are offered genetic counselling, which includes the possibility of presymptomatic DNA testing. However, this is only offered after a series of careful counselling sessions to ensure that the individual understands the issues involved in knowing their genetic status.
- TOMM40
-
A gene encoding a mitochondrial protein that is located on chromosome 19 adjacent to the apolipoprotein E gene.
- Risk chart
-
A graph illustrating the risk of developing Alzheimer's disease by age. This risk is modified by genetic status, especially by apolipoprotein E genotype status. Other genes will have a smaller effect on this chart (except in families with amyloid precursor protein and presenilin mutations).
- Phase 0 trial
-
An exploratory first-in-human trial with single subtherapeutic drug doses and small numbers of subjects to provide first data on drug pharmacokinetics and pharmacodynamics.
Rights and permissions
About this article
Cite this article
Hampel, H., Frank, R., Broich, K. et al. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 9, 560–574 (2010). https://doi.org/10.1038/nrd3115
Issue Date:
DOI: https://doi.org/10.1038/nrd3115
This article is cited by
-
miR-129-5p as a biomarker for pathology and cognitive decline in Alzheimer’s disease
Alzheimer's Research & Therapy (2024)
-
Gene expression patterns of CRYM and SIGLEC10 in Alzheimer's disease: potential early diagnostic indicators
Molecular Biology Reports (2024)
-
Targeting the “hallmarks of aging” to slow aging and treat age-related disease: fact or fiction?
Molecular Psychiatry (2023)
-
Advanced Overview of Biomarkers and Techniques for Early Diagnosis of Alzheimer’s Disease
Cellular and Molecular Neurobiology (2023)
-
Application of 3D Whole-Brain Texture Analysis and the Feature Selection Method Based on within-Class Scatter in the Classification and Diagnosis of Alzheimer’s Disease
Therapeutic Innovation & Regulatory Science (2022)