Key Points
-
Germline mutations in the genes that encode the BMP/TGF-β superfamily are associated with heritable vascular disorders and cancer syndromes.
-
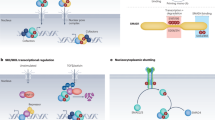
The TGF-β pathway involves the heterodimerization of type II and type I receptors after ligand binding, which leads to heterodimerization of R-SMAD and Co-SMAD. The SMAD complex translocates to the nucleus, where it regulates transcription.
-
Germline mutations in BMPR2, which result in haploinsufficiency, cause primary pulmonary hypertension (PPH). BMPR2 encodes BMPR-II, which is a type II receptor in the TGF-β pathway. Most of these mutations occur in the ligand- or kinase-binding domains and disrupt the signal-transducing abilities of the receptor.
-
Germline mutations in ENG, which encodes endoglin (a co-receptor for type II receptors), are associated with hereditary haemorrhagic telangiectasia 1 (HHT1). Germline mutations cause haploinsufficiency or dominant-negative protein expression.
-
Germline mutations in ALK1, which encodes the type I receptor ALK1, are associated with HHT2. Mutations in ALK1 are predicted to cause truncated proteins and haploinsufficiency.
-
Germline mutations in either MADH4, which encodes SMAD4, or BMPR1A, which encodes the type IA receptor for BMP2, are involved in juvenile polyposis syndrome. Mutations in MADH4 prevent the heterodimerization of SMAD4 with R-SMADs, whereas mutations in BMPR1A result in the loss of ligand binding or kinase activity.
-
BMPR1A is a minor susceptibility gene for Cowden syndrome, which is a hamartoma-cancer syndrome.
-
The BMP and PTEN pathways might crosstalk.
Abstract
Transforming growth factor-β (TGF-β) regulates many cellular processes through complex signal-transduction pathways that have crucial roles in normal development. Disruption of these pathways can lead to a range of diseases, including cancer. Mutations in the genes that encode members of the TGF-β pathway are involved in vascular diseases as well as gastrointestinal neoplasia. More recently, they have been implicated in Cowden syndrome, which is normally associated with mutations in the phosphatase and tensin homologue gene PTEN. Molecular studies of TGF-β signalling are now showing why mutations in genes that encode components of this pathway result in inherited cancer and developmental diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Markowitz, S. et al. Inactivation of the type II TGF-B receptor in colon cancer cells with microsatellite instability. Science 268, 1336–1338 (1995).
Teicher, B. A. Malignant cells, directors of the malignant process: role of transforming growth factor-β. Cancer Met. Rev. 20, 133–143 (2001).
Miyazono, K. TGF-B/SMAD signaling and its involvement in tumor progression. Biol. Pharm. Bull. 23, 1125–1130 (2000).
Miyazono, K. TGF-B signaling by Smad proteins. Cytokine Growth Fact. Rev. 11, 15–22 (2000).
Zimmerman, C. M. & Padgett, R. W. Transforming growth factor-β signaling mediators and modulators. Gene 249, 17–30 (2000).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nature Genet. 29, 117–129 (2001).
Shi, Y. & Massague, J. Mechanisms of TGF-β signaling: from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Nichols, W. C. et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31-q33. Nature Genet. 15, 277–280 (1997).
Morse, J. H. et al. Mapping of familial primary pulmonary hypertension locus (PPH1) to chromosome 2q31-q32. Circulation 95, 2603–2606 (1997).
Machado, R. D. et al. A physical and transcript map based upon refinement of the critical interval for PPH1, a gene for familial primary pulmonary hypertension. Genomics 68, 220–228 (2000).
International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGFβ receptor, cause familial primary pulmonary hypertension. Nature Genet. 26, 81–84 (2000). This study identifies BMPR2 as the susceptibility gene for familial primary pulmonary hypertension.
Deng, Z. et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am. J. Hum. Genet. 67, 737–744 (2000).
Machado, R. D. et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am. J. Hum. Genet. 68, 92–102 (2001).
Thomson, J. R. et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-B family. J. Med. Genet. 37, 741–745 (2000). This paper reports that ∼25% of apparently sporadic cases of primary pulmonary hypertension carry unsuspected germline mutations in BMPR2.
Eng, C. et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2: International RET Mutation Consortium analysis. JAMA 276, 1575–1579 (1996).
Dorai, H., Vukecevic, S. & Sampath, T. K. Bone morphogenetic protein-7 (Op-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J. Cell. Physiol. 184, 37–45 (2000).
Nakaoka, T. et al. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by morphogenetic protein-2. J. Clin. Invest. 100, 2824–2832 (1997).
Morrell, N. W. et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-B1 and bone morphogenetic proteins. Circulation 104, 790–795 (2001).
Atkinson, C. et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105, 1672–1678 (2002).
Rudarakanchana, N. et al. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum. Mol. Genet. 11, 1517–1525 (2002).
Nishihara, A., Watabe, T., Imamura, T. & Miyazono, K. Functional heterogeneity of bone morphogenetic protein receptor-II mutants found in patients with primary pulmonary hypertension. Mol. Biol. Cell 13, 3055–3063 (2002).
Massague, J. How cells read TGF-β signals. Nature Rev. Mol. Cell. Biol. 1, 169–178 (2000).
Roberts, A. B. The ever-increasing complexity of TGF-β signaling. Cytokine Growth Fact. Rev. 13, 3–5 (2002).
Eng, C. To be or not to BMP. Nature Genet. 28, 105–107 (2001).
Eddahibi, S. et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J. Clin. Invest. 108, 1141–1150 (2001).
Porteus, M. E. M., Burn, J. & Proctor, S. Hereditary haemorrhagic telangeictasia: a clinical analysis. J. Med. Genet. 29, 527–530 (1992).
Guttmacher, A. E., Marchuk, D. A. & White, R. I. Hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 333, 918–924 (1995).
White, R. I. & Pollak, J. S. Pulmonary arteriovenous malformations: options for management. Ann. Thorac. Surg. 57, 519–521 (1994).
Shovlin, C. L. et al. A gene for hereditary haemorrhagic telangiectasia maps to chromosome 9q. Nature Genet. 6, 205–209 (1994).
Vincent, P. et al. A third locus for hereditary haemorrhagic telangiectasia maps to chromosome 12q. Hum. Mol. Genet. 4, 945–949 (1995).
Johnson, D. W. et al. A second locus for hereditary haemorrhagic telangiectasia maps to chromosome 12. Genome Res. 5, 21–28 (1995).
McAllister, K. A. et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nature Genet. 8, 345–351 (1994). This study identifies ENG -encoding endoglin as a susceptibility gene for hereditary haemorrhagic telangiectasia.
Johnson, D. W. et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nature Genet. 13, 189–196 (1996). In this study, ALK1 (ACVRL1 ) is identified as a second susceptibility gene for hereditary haemorrhagic telangiectasia.
Shovlin, C. L., Hughes, J. M. B., Scott, J., Seidman, C. E. & Seidman, J. G. Characterization of endoglin and identification of novel mutations in hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet. 61, 68–79 (1997).
Berg, J. N., Guttmacher, A. E., Marchuk, D. A. & Porteous, M. E. M. Clinical heterogeneity in hereditary haemorrhagic telangiectasia — are pulmonary arteriovenous malformations more common in families linked to endoglin? J. Med. Genet. 33, 256–257 (1996).
Dakeishi, M. et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum. Mutat. 19, 140–148 (2002).
Gallione, C. J. et al. Two common endoglin mutations in families with hereditary hemorrhagic telangiectasia in the Netherlands Antilles: evidence for a founder effect. Hum. Genet. 107, 40–44 (2000).
Berg, J. N. et al. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am. J. Hum. Genet. 61, 60–67 (1997).
Abdalla, S. A., Cymerman, U., Johnson, R. M., Deber, C. M. & Letarte, M. Disease-associated mutations in conserved residues of ALK-1 kinase domain. Eur. J. Hum. Genet. 11, 279–287 (2003).
Hanks, S. K., Quinn, A. M. & Hunter, T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domain. Science 241, 42–52 (1988).
Garamszegi, N. et al. Transforming growth factor-β receptor signaling and endocytosis are linked through a COOH terminal activation motif in the type 1 receptor. Mol. Biol. Cell 12, 2881–2893 (2001).
Guerreoro-Esteo, M., Sanchez-Elsner, T., Letamendia, A. & Bernabeu, C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-β receptors I and II. J. Biol. Chem. 277, 29197–29209 (2002).
Lastres, P. et al. Endoglin modulates cellular responses to TGF-β1. J. Cell. Biol. 133, 1109–1121 (1996).
Barbara, N. P., Wrana, J. L. & Letarte, M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor B superfamily. J. Biol. Chem. 274, 584–594 (1999).
Pece, S. et al. Mutant endoglin in hereditary hemorrhagic telangiectasia type 1 is transiently expressed intracellularly and is not a dominant negative. J. Clin. Invest. 100, 2568–2579 (1997).
Raab, U. et al. Expression of normal and truncated forms of human endoglin. Biochem. J. 339, 579–588 (1999).
Lux, A., Gallione, C. J. & Marchuk, D. A. Expression analysis of endoglin missense and truncation mutations: insights into protein structure and disease mechanisms. Hum. Mol. Genet. 22, 745–755 (2000).
Paquet, M. E. et al. Analysis of several endoglin mutants reveals no endogenous mature or secreted protein capable of interfering with normal endoglin function. Hum. Mol. Genet. 10, 1347–1357 (2001).
Bourdeau, A., Dumont, D. J. & Letarte, M. A. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Invest. 104, 1343–1351 (1999).
Bourdeau, A. et al. Potential role of modifier genes influencing transforming growth factor-B1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am. J. Pathol. 158, 2011–2020 (2001).
Santomi, J. et al. Cerebral vascular abnormalities in a murine model for hereditary hemorrhagic telangiectasia. Stroke 34, 783–789 (2003).
Fanning, A. S. & Anderson, J. M. Protein modules as organizers of membrane structure. Curr. Opin. Cell Biol. 432–439 (1999).
Kay, B. K., Williamson, M. P. & Sudol, M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14, 231–241 (2000).
Howe, J. R. et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science 280, 1086–1088 (1998). This study identifies MADH4 as a susceptibility gene for a subset of families with juvenile polyposis syndrome.
Howe, J. R. et al. Germline mutations of BMPR1A in juvenile polyposis. Nature Genet. 28, 184–187 (2001). In this study, BMPR1A germline mutations are identified in MADH4 -mutation negative probands with familial juvenile polyposis syndrome.
Zhou, X. P. et al. Germline mutations in BMPR1A/ALK3 cause a subset of juvenile polyposis syndrome and of Cowden and Bannayan–Riley–Ruvalcaba syndromes. Am. J. Hum. Genet. 69, 704–711 (2001). This paper reports that germline BMPR1A mutations cause sporadic juvenile polyposis syndrome as well as rare Cowden syndrome probands.
Eng, C. & Blackstone, M. O. Peutz–Jeghers syndrome. Med. Rounds 1, 165–171 (1988).
Jarvinen, J. & Franssila, K. O. Familial juvenile polyposis coli: increased risk of colorectal cancer. Gut 25, 792–800 (1984).
Coburn, M. C., Pricolo, V. E., DeLuca, F. G. & Bland, K. I. Malignant potential in intestinal juvenile polyposis syndromes. Ann. Surg. Oncol. 2, 386–391 (1995).
Howe, J. R., Mitros, F. A. & Summers, R. W. The risk of gastrointestinal carcinoma in familial juvenile polyposis. Ann. Surg. Oncol. 5, 751–756 (1998).
Hofting, I., Pott, G. & Stolte, M. Das syndrom der juvenilen polyposis. Leber Magen. Darm. 23, 107–108 (1993).
Howe, J. R. et al. Common deletion of SMAD4 in juvenile polyposis is a mutational hotspot. Am. J. Hum. Genet. 70, 1357–1362 (2002).
Friedl, W. et al. Juvenile polyposis: massive gastric polyposis is more common in MADH4 mutation carriers than in BMPR1A mutation carriers. Hum. Genet. 111, 108–111 (2002). This study shows that MADH4 -mutation positive juvenile polyposis probands are at increased risk of giant gastric polyps compared with those that have BMPR1A mutations.
Sayed, M. G. et al. Germline SMAD4 or BMPR1A mutations and phenotype in juvenile polyposis. Ann. Surg. Oncol. 9, 901–906 (2002).
Shi, Y., Hata, A., Lo, R. S., Massague, J. & Pavletich, N. P. A structural basis for the mutational inactivation of the tumor suppressor Smad4. Nature 388, 87–93 (1997).
Kawabata, M., Inoue, H., Hanyu, A., Imamura, T. & Miyazono, K. SMAD proteins exist as monomers in vivo and undergo homo- and hetero-oligomerisation upon activation by serine/threonine kinase receptors. EMBO J. 17, 4056–4065 (1998).
Bevan, S. et al. Screening SMAD1, SMAD2, SMAD3 and SMAD5 for germline mutations in juvenile polyposis syndrome. Gut 45, 406–408 (1999).
Olschwang, S., Serova-Sinilnikova, O. M., Lenoir, G. M. & Thomas, G. PTEN germline mutations in juvenile polyposis coli. Nature Genet. 18, 12–14 (1998).
Eng, C. & Ji, H. Molecular classification of the inherited hamartoma polyposis syndromes: clearing the muddied waters. Am. J. Hum. Genet. 62, 1020–1022 (1998).
Kurose, K., Araki, T., Matsunaka, T., Takada, Y. & Emi, M. Variant manifestation of Cowden disease in Japan: hamartomatous polyposis of the digestive tract with mutation of the PTEN gene. Am. J. Hum. Genet. 64, 308–310 (1999).
Eng, C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J. Med. Genet. 37, 828–830 (2000).
Waite, K. A. & Eng, C. BMP2 exposure results in decreased PTEN protein degradation leading to increased PTEN levels. Hum. Mol. Genet. 12, 679–684 (2003). This study shows that BMP decreases PTEN protein degradation, which results in increased PTEN levels. Therefore, by implication, BMP signalling can result in apoptosis that is mediated by PTEN.
Trembath, R. C. et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 345, 325–334 (2001).
Blanquet, V., Créau-Goldberg, N., de Grouchy, J. & Turleau, C. Molecular detection of constitutional deletions in patients with retinoblastoma. Am. J. Med. Genet. 39, 355–361 (1991).
Blanquet, V. et al. Spectrum of germline mutations in the RB1 gene: a study of 232 patients with hereditary and non hereditary retinoblastoma. Hum. Mol. Genet. 4, 383–388 (1995).
Stolle, C. et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum. Mutat. 12, 417–423 (1998).
Zhou, X. P. et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan–Riley–Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am. J. Hum. Genet. (in the press).
Abraham, W. T. et al. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: increased frequency and association with preserved haemodynamics. J. Renin Angiotens. Aldoster. Sys. 4, 27–30 (2003).
Weber, H. C., Marsh, D., Lubensky, I., Lin, A. & Eng, C. Germline PTEN/MMAC1/TEP1 mutations and association with gastrointestinal manifestations in Cowden disease. Gastroenterology 114, 2902 (1998).
Hemminki, A. et al. Localisation of a susceptibility locus for Peutz–Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nature Genet. 15, 87–90 (1997).
Hemminki, A. et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391, 184–187 (1998).
Jenne, D. E. et al. Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nature Genet. 18, 38–44 (1998).
Mehenni, H. et al. Peutz–Jeghers syndrome: confirmation of linkage to chromosome 19p13.3 and identification of a potential second locus on 19q13.4. Am. J. Hum. Genet. 61, 1327–1334 (1997).
Liaw, D. et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature Genet. 16, 64–67 (1997).
Marsh, D. J. et al. Mutation spectrum and genotype–phenotype analyses in Cowden disease and Bannayan–Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum. Mol. Genet. 7, 507–515 (1998).
Marsh, D. J. et al. Germline mutations in PTEN are present in Bannayan–Zonana syndrome. Nature Genet. 16, 333–334 (1997).
Marsh, D. J. et al. PTEN mutation spectrum and genotype–phenotype correlations in Bannayan–Riley–Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum. Mol. Genet. 8, 1461–1472 (1999).
Jaeger, E. E. M. et al. An ancestral Ashkenazi haplotype at the HMPS/CRAC1 locus on 15q13-q14 is associated with hereditary mixed polyposis syndrome. Am. J. Hum. Genet. 72, 1261–1267 (2003).
van den Driesche, S., Mummery, C. L. & Westermann, C. J. Hereditary hemorrhagic telangiectasia: an update on transforming growth factor-β signaling in vasculogenesis and angiogenesis. Cardiovasc. Res. 58, 20–31 (2003).
Acknowledgements
We are grateful to J. Howe for sharing pre-publication data, and to L. Aaltonen, W. Friedl and R. Trembath for helpful discussions. C.E. is the recipient of a Doris Duke Distinguished Clinical Scientist Award and is partially funded by the American Cancer Society, the Department of Defense United States Army Breast and Prostate Cancer Research Programs, the Susan G. Komen Breast Cancer Research Foundation, the National Cancer Institute, the National Institutes of Health, the State of Ohio Biomedical Research and Technology Transfer Fund and the V Foundation Jimmy V Golf Classic Translational Cancer Research Award.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez
LocusLink
OMIM
Bannayan–Riley–Ruvalcaba syndrome
hereditary haemorrhagic telangiectasia
hereditary mixed polyposis syndrome
multiple endocrine neoplasia type 2
primary pulmonary hypertension
PTEN hamartoma tumour syndrome
FURTHER INFORMATION
Genomic Disorders Research Centre
Human Genome Variation Society Nomenclature for the Description of Sequence Variations
Glossary
- HAEMATOPOIESIS
-
The development of blood cells.
- ARTERIOLES
-
Small arteries. Blood flows from arteries to arterioles to capillaries, and returns to the heart through venules (small veins) and then veins.
- RIGHT HEART FAILURE
-
Heart failure that is limited to the right-sided chambers of the heart, which is usually caused by lung problems.
- LOCUS HETEROGENEITY
-
Refers to an inherited disorder with more than one susceptibility gene.
- HAPLOINSUFFICIENCY
-
The half dosage of a gene product, which leads to an adverse phenotype.
- SDS-PAGE
-
Sodium dodecyl sulphate-polyacrimide gel electrophoresis. A rapid and inexpensive method for resolving a protein into its subunits and determining their relative molecular masses.
- MULTI-SYSTEM VASCULAR DYSPLASIA
-
The atypical growth of vessels in many organs.
- TELANGIECTASIAS
-
Dilated capillaries and arterioles.
- FOUNDER EFFECT
-
A mutation that originated in a single individual, which has been passed on through the generations and spread through the population.
- MESENCHYMAL
-
(Stromal). Cells that do not form gap junctions with one another.
- MODIFIER GENES
-
Variants in these genes themselves do not produce a phenotype, but when they are present with other variants of germline mutations they produce a phenotypic effect.
- INTERSUSSCEPTION
-
The telescoping of the bowel on itself.
- HAMARTOMATOUS POLYPS
-
Hamartomas are benign developmentally incorrect overgrowths of any tissue that comprise at least two elements of the tissue. Hamartomatous polyps are outgrowths in the gastrointestinal tract, which comprise at least two components of the normal intestine but in developmentally incorrect apposition. They are not considered to be pre-neoplastic.
- PROBANDS
-
The first known affected individual in any given family.
- ENDOMETRIAL CARCINOMAS
-
Cancer of the inner-lining of the uterus.
- LIPOMATOSIS
-
Multiple fatty tumours.
- POLYPECTOMIES
-
The removal of polyps.
- PATHOGNOMONIC
-
Absolutely diagnostic.
- HAEMODYNAMICS
-
The physics of all aspects of blood flow in large and small arteries.
Rights and permissions
About this article
Cite this article
Waite, K., Eng, C. From developmental disorder to heritable cancer: it's all in the BMP/TGF-β family. Nat Rev Genet 4, 763–773 (2003). https://doi.org/10.1038/nrg1178
Issue Date:
DOI: https://doi.org/10.1038/nrg1178
This article is cited by
-
Tumor cell-derived ANGPTL2 promotes β-catenin-driven intestinal tumorigenesis
Oncogene (2022)
-
The impact of anti-TNF treatment on Wnt signaling, noggin, and cytokine levels in axial spondyloarthritis
Clinical Rheumatology (2022)
-
EMP2 induces cytostasis and apoptosis via the TGFβ/SMAD/SP1 axis and recruitment of P2RX7 in urinary bladder urothelial carcinoma
Cellular Oncology (2021)
-
Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients
Journal of Translational Medicine (2020)
-
Familial juvenile polyposis syndrome with a de novo germline missense variant in BMPR1A gene: a case report
BMC Medical Genetics (2020)