Key Points
-
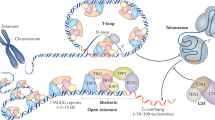
Telomeres are specialized chromatin structures located at the ends of chromosomes that protect chromosome ends from repair and degradation activities. Telomeres consist of G-rich repeats, which are bound by telomere-repeat-binding factors.
-
Telomerase is a cellular reverse transcriptase that synthesizes telomeric repeats de novo at chromosome ends. Recombination between telomeric sequences can also lead to telomere elongation independently of telomerase.
-
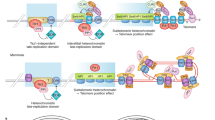
Telomeres and subtelomeres contain histone and DNA modifications that are also enriched at constitutive heterochromatin domains, such as those of pericentric heterochromatin.
-
From yeast to mammals, loss of heterochromatic marks at telomeres and subtelomeres results in telomere-length deregulation and disruption of telomeric silencing, or TPE (the transcriptional repression of genes located near the telomeres).
-
Loss of either histone methylation or DNA methylation at mammalian telomeres or subtelomeres also leads to de-repression of telomere recombination.
-
Histone- and DNA-methylation defects are associated with several human diseases, including cancer. These defects could have an impact on telomere-length regulation, and therefore contribute to disease phenotypes.
-
Telomere shortening to a critically short length leads to epigenetic defects at mammalian telomeres and subtelomeres, characterized by decreased histone and DNA methylation and increased histone acetylation.
-
Histone and DNA modifications provide a mechanism by which telomere repeats are counted and autoregulated.
-
Various diseases associated with ageing, including cancer and a number of premature ageing syndromes, are characterized by critically short telomeres, which in turn could affect the epigenetic status of telomeres and subtelomeres.
Abstract
Increasing evidence indicates that chromatin modifications are important regulators of mammalian telomeres. Telomeres provide well studied paradigms of heterochromatin formation in yeast and flies, and recent studies have shown that mammalian telomeres and subtelomeric regions are also enriched in epigenetic marks that are characteristic of heterochromatin. Furthermore, the abrogation of master epigenetic regulators, such as histone methyltransferases and DNA methyltransferases, correlates with loss of telomere-length control, and telomere shortening to a critical length affects the epigenetic status of telomeres and subtelomeres. These links between epigenetic status and telomere-length regulation provide important new avenues for understanding processes such as cancer development and ageing, which are characterized by telomere-length defects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005). A must-read review on the protein composition of mammalian telomeres and their role in the regulation of telomere length and telomere capping.
Chan, S. W. & Blackburn, E. H. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 21, 553–563 (2002).
Liu, D., O'Connor, M. S., Qin, J. & Songyang, Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J. Biol. Chem. 279, 51338–51342 (2004).
Collins, K. & Mitchell, J. R. Telomerase in the human organism. Oncogene 21, 564–579 (2002).
Muntoni, A. & Reddel, R. R. The first molecular details of ALT in human tumor cells. Hum. Mol. Genet. 14, 191–196 (2005).
Dunham, M. A., Neumann, A. A., Fasching, C. L. & Reddel, R. R. Telomere maintenance by recombination in human cells. Nature Genet. 26, 447–450 (2000).
Slagboom, P. E., Droog, S. & Boomsma, D. I. Genetic determination of telomere size in humans: a twin study of three age groups. Am. J. Hum. Genet. 55, 876–882 (1994).
Zhu, L. et al. Telomere length regulation in mice is linked to a novel chromosome locus. Proc. Natl Acad. Sci. USA 95, 8648–8653 (1998).
Flores, I., Cayuela, M. L. & Blasco, M. A. Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256 (2005).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Lee, H.-W., Blasco, M. A., Gottlieb, G. J., Greider, C. W. & DePinho, R. A. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Herrera, E. et al. Disease states associated to telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Mason, P. J., Wilson, D. B. & Bessler, M. Dyskeratosis congenita — a disease of dysfunctional telomere maintenance. Curr. Mol. Med. 5, 159–170 (2005).
Shay, J. W. & Wright, W. E. Telomerase therapeutics for cancer: challenges and new directions. Nature Rev. Drug Discov. 5, 577–584 (2006).
Baur, J. A., Zou, Y., Shay, J. W. & Wright, W. E. Telomere position effect in human cells. Science 292, 2075 (2001).
Koering, C. E. et al. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3, 1055–1061 (2002). References 15 and 16 provide conclusive evidence that TPE (or 'silencing' of genes near the telomeres) operates in mammalian cells.
Makarov, V. L., Lejnine, S., Bedoyan, J. & Langmore, J. P. Nucleosomal organization of telomere-specific chromatin in rat. Cell 73, 775–787 (1993).
Tommerup, H., Dousmanis, A. & de Lange, T. Unusual chromatin in human telomeres. Mol. Cell. Biol. 14, 5777–5785 (1994).
Garcia-Cao, M., O'Sullivan, R., Peters, A. H., Jenuwein, T. & Blasco, M. A. Epigenetic regulation of telomere length in mammalian cells by the SUV39H1 and SUV39H2 histone methyltransferases. Nature Genet. 36, 94–99 (2004).
Gonzalo, S. et al. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nature Cell Biol. 7, 420–428 (2005).
Gonzalo, S. et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nature Cell Biol. 8, 416–424 (2006). This work shows that mammalian subtelomeric DNA is heavily methylated, and that this epigenetic modification acts as a negative regulator of telomere length and telomere recombination independently of histone methylation.
Fraga, M. F. et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nature Genet. 37, 391–400 (2005).
van Overveld, P. G. et al. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nature Genet. 35, 315–317 (2003).
Garcia-Cao, M., Gonzalo, S., Dean, D. & Blasco, M. A. A role for the Rb family of proteins in controlling telomere length. Nature Genet. 32, 415–419 (2002).
Blasco, M. A. Telomeres and human disease: ageing, cancer and beyond. Nature Rev. Genet. 6, 611–622 (2005).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
de Lange, T. T-loops and the origin of telomeres. Nature Rev. Mol. Cell Biol. 5, 323–329 (2004).
Conrad, M. N., Wright, J. H., Wolf, A. J. & Zakian, V. A. Rap1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell 63, 739–750 (1990).
Nugent, C. I., Hughes, T. R., Lue, N. F. & Lundblad, V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274, 249–252 (1996).
Tham, W. H. & Zakian, V. A. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene 21, 512–521 (2002). A must-read review on budding yeast telomeric heterochromatin and its roles in controlling telomere length and telomeric silencing.
Marcand, S., Gilson, E. & Shore, D. A protein-counting mechanism for telomere length regulation in yeast. Science 275, 986–990 (1997).
Kyrion, G., Boakye, K. A. & Lustig, A. J. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol. Cell. Biol. 12, 5159–5173 (1992).
Krauskopf, A. & Blackburn, E. H. Control of telomere growth by interactions of RAP1 with the most distal telomeric repeats. Nature 383, 354–357 (1996).
Levy, D. L. & Blackburn, E. H. Counting of Rif1p and Rif2p on Saccharomyces cerevisiae telomeres regulates telomere length. Mol. Cell. Biol. 24, 10857–10867 (2004).
Palladino, F. et al. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell 75, 543–555 (1993).
Cooper, J. P., Nimmo, E. R., Allshire, R. C. & Cech, T. R. Regulation of telomere length and function by a MYB-domain protein in fission yeast. Nature 385, 744–747 (1997).
Baumann, P. & Cech, T. R. POT1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Kanoh, J. & Ishikawa, F. spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr. Biol. 11, 1624–1630 (2001).
Ye, J. Z. et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004).
Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nature Cell Biol. 6, 673–680 (2004).
Smith, S., Giriat, I., Schmitt, A. & de Lange, T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282, 1484–1487 (1998).
Celli, G. B. & de Lange, T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nature Cell Biol. 7, 712–718 (2005).
Hockemeyer, D., Daniela, J. P., Takai, H. & de Lange, T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell 126, 63–77 (2006).
Zhu, X. D., Kuster, B., Mann, M., Petrini, J. H. & Lange, T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nature Genet. 25, 347–352 (2000).
Samper, E., Goytisolo, F. A., Slijepcevic, P., van Buul, P. P. & Blasco, M. A. Mammalian KU86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep. 1, 244–252 (2000).
Tarsounas, M. et al. Telomere maintenance requires the RAD51D recombination/repair protein. Cell 117, 337–347 (2004).
Karlseder, J. et al. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2, e240 (2004).
Bradshaw, P. S., Stavropoulos, D. J & Meyn, M. S. Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nature Genet. 37, 193–197 (2005).
Oh, B.-K., Kim, Y.-J., Park, C. & Park, Y. N. Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am. J. Pathol. 166, 73–80 (2005).
Matsutani, N. et al. Expression of telomeric repeat binding factor 1 and 2 and TRF1-interacting nuclear protein 2 in human gastric carcinomas. Int. J. Oncol. 19, 507–512 (2001).
Muñoz, P. et al. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nature Genet. 10, 1063 (2005).
Nakanishi, K. et al. Expression of mRNAs for telomeric repeat binding factor (TRF)-1 and TRF2 in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Clin. Cancer Res. 9, 1105–1111 (2003).
Bellon, M. et al. Increased expression of telomere length regulating factors TRF1, TRF2 and TIN2 in patients with adult T-cell leukemia. Int. J. Cancer 119, 2090–2097 (2006).
Blanco, R., Muñ oz, P., Klatt, P., Flores, J. M. & Blasco, M. A. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 21, 206–220 (2007).
Lazzerini Denchi, E., Celli, G. & de Lange, T. Hepatocytes with extensive telomere deprotection and fusion remain viable and regenerate liver mass through endoreduplication. Genes Dev. 20, 2648–2653 (2006).
Flores, I., Cayuela, M. L. & Blasco, M. A. Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256 (2005).
Sarin, K. Y. et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048–1052 (2005).
Mason, J. M. & Biessmann, H. The unusual telomeres of Drosophila. Trends Genet. 11, 58–62 (1995).
Lundblad, V. & Blackburn, E. H. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell 73, 347–360 (1993).
Lundblad, V. Telomere maintenance without telomerase. Oncogene 21, 522–531 (2002).
Le, S., Moore, J. K., Haber, J. E. & Greider, C. W. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics 152, 143–152 (1999).
Teng, S. C. & Zakian, V. A. Telomere–telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 8083–8093 (1999).
Hande, M. P., Samper, E., Lansdorp, P. & Blasco, M. A. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J. Cell Biol. 44, 589–601 (1999).
Chang, S., Khoo, C. M., Naylor, M. L., Maser, R. S. & DePinho, R. A. Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 17, 88–100 (2003).
Niida, H. et al. Telomere maintenance in telomerase-deficient mouse embryonic stem cells: characterization of an amplified telomeric DNA. Mol. Cell. Biol. 20, 4115–4127 (2000).
Herrera, E., Martinez, A. C. & Blasco, M. A. Impaired germinal center reaction in mice with short telomeres. EMBO J. 19, 472–481 (2000).
Laud, P. R. et al. Elevated telomere–telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 19, 2560–2570 (2005).
Wu, L. et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126, 49–62 (2006).
Levis, R., Hazelrigg, T. & Rubin, G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science 229, 558–561 (1985).
Gottschling, D. E., Aparicio, O. M., Billington, B. L. & Zakian, V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–762 (1990).
Nimmo, E. R., Cranston, G. & Allshire, R. C. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 13, 3801–3811 (1994).
Kyrion, G., Liu, K., Liu, C. & Lustig, A. J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 7, 1146–1159 (1993).
Aparicio, O. M., Billington, B. L. & Gottschling, D. E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66, 1279–1287 (1991).
Hecht, A., Laroche, T., Strahl-Bolsinger, S., Gasser, S. M. & Grunstein, M. Histone H3 and H4 N-termini interact with Sir3 and Sir4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80, 583–592 (1995). A molecular model for how heterochromatin is formed at budding yeast telomeres is first proposed here.
Moretti, P. & Shore, D. Multiple interactions in Sir protein recruitment by Rap1p at silencers and telomeres in yeast. Mol. Cell. Biol. 21, 8082–8094 (2001).
Wright, J. H., Gottschling, D. E. & Zakian, V. A. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes Dev. 6, 197–210 (1992).
Luo, K., Vega-Palas, M. A. & Grunstein, M. Rap1–Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 16, 1528–1539 (2002).
Strahl-Bolsinger, S., Hecht, A., Luo, K. & Grunstein, M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 11, 83–93 (1997).
de Bruin, D., Kantrow, S. M., Liberatore, R. A. & Zakian, V. A. Telomere folding is required for the stable maintenance of telomere position effects in yeast. Mol. Cell. Biol. 20, 7991–8000 (2000).
Maillet, L. et al. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 10, 1796–1811 (1996).
Fourel, G., Revardel, E., Koering, C. E. & Gilson, E. Cohabitation of insulators and silencing elements in yeast subtelomeric regions. EMBO J. 18, 2522–2537 (1999).
Pryde, F. E. & Louism, E. J. Limitations of silencing at native yeast telomeres. EMBO J. 18, 2538–2550 (1999).
Emre, N. C. et al. Maintenance of low histone ubiquitylation by Ubp10 correlates with telomere-proximal Sir2 association and gene silencing. Mol. Cell 17, 585–594 (2005).
Cenci, G. et al. UbcD1, a Drosophila ubiquitin-conjugating enzyme required for proper telomere behavior. Genes Dev. 11, 863–875 (1997).
Greenwell, P. W. et al. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82, 823–829 (1995).
Porter, S. E., Greenwell, P. W., Ritchie, K. B. & Petes, T. D. The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res. 24, 582–585 (1996).
Nislow, C., Ray, E. & Pillus, L. SET1, a yeast member of the trithorax family, functions in transcriptional silencing and diverse cellular processes. Mol. Biol. Cell 8, 2421–2436 (1997).
Gartenberg, M. R., Neumann, F. R., Laroche, T., Blaszczyk, M. & Gasser, S. M. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119, 955–967 (2004).
Berthiau, A. S. et al. Subtelomeric proteins negatively regulate telomere elongation in budding yeast. EMBO J. 25, 846–856 (2006).
Hediger, F., Berthiau, A. S., van Houwe, G., Gilson, E. & Gasser, S. M. Subtelomeric factors antagonize telomere anchoring and Tel1-independent telomere length regulation. EMBO J. 25, 857–867 (2006).
Teng, S. C., Chang, J., McCowan, B. & Zakian, V. A. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol. Cell 6, 947–952 (2000).
Nakayama, J., Rice, J. C., Strahl, B. D., Allis, C. D. & Grewal, S. I. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113 (2001).
Volpe, T. A. et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002).
Kanoh, J., Sadaie, M., Urano, T. & Ishikawa, F. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr. Biol. 15, 1808–1819 (2005).
Ueno, M. et al. Fission yeast Arp6 is required for telomere silencing, but functions independently of Swi6. Nucleic Acids Res. 32, 736–741 (2004).
Ekwall, K. et al. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localisation of the chromo domain protein Swi6p and impair centromere function. J. Cell Sci. 109, 2637–2648 (1996).
Hall, I. M., Noma, K. & Grewal, S. I. RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc. Natl Acad. Sci. USA 100, 193–198 (2003).
Perrini, B. et al. HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol. Cell 15, 467–476 (2004).
Savitsky, M., Kwon, D., Georgiev, P., Kalmykova, A. & Gvozdev, V. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 20, 345–354 (2006).
Xhemalce, B., Seeler, J. S., Thon, G., Dejean, A. & Arcangioli, B. Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J. 23, 3844–3853 (2004).
Xhemalce, B. et al. Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc. Natl Acad. Sci. USA. 104, 893–898 (2007). A role for SUMO proteins in telomerase-mediated telomere elongation is first shown here.
Kanoh, J. et al. The fission yeast spSet1p is a histone H3-K4 methyltransferase that functions in telomere maintenance and DNA repair in an ATM kinase Rad3-dependent pathway. J. Mol. Biol. 326, 1081–1094 (2003).
Peters, A. H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Schotta, G. et al. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev 18, 1251–1262 (2004).
Kourmouli, N. et al. Heterochromatin and tri-methylated lysine 20 of histone 4 in mammals. J. Cell Sci. 117, 2491–2501 (2004).
Lachner, M., O'Carroll, D., Rea, S., Mechtler, K. & Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 (2001).
Benetti, R., Garcia-Cao, M. & Blasco, M. A. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nature Genet. 39, 243–250 (2007). Shows for the first time that telomere shortening to a critical length in mammals results in loss of histone and DNA methylation at mammalian telomeres and subtelomeres, concomitant with increased histone acetylation.
Netzer, C. et al. SALL1, the gene mutated in Townes–Brocks syndrome, encodes a transcriptional repressor which interacts with TRF1/PIN2 and localizes to pericentromeric heterochromatin. Hum. Mol. Genet. 10, 3017–3024 (2001).
Kaminker, P. et al. Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2. J Cell Sci. 118, 1321–1330 (2005).
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nature Rev. Genet. 3, 415–428 (2002).
Dominguez-Bendala, J. & McWhir, J. Enhanced gene targeting frequency in ES cells with low genomic methylation levels. Transgenic Res. 13, 69–74 (2004).
Maloisel, L. & Rossignol, J. L. Suppression of crossing-over by DNA methylation in Ascobolus. Genes Dev. 12, 1381–1389 (1998).
Bender, J. Cytosine methylation of repeated sequences in eukaryotes: the role of DNA pairing. Trends Biochem. Sci. 23, 252–256 (1998).
Pedram, M. et al. Telomere position effect and silencing of transgenes near telomeres in the mouse. Mol. Cell. Biol. 26, 1865–1878 (2006).
Steinert, S., Shay, J. W. & Wright, W. E. Modification of subtelomeric DNA. Mol. Cell. Biol. 24, 4571–4580 (2004).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases DNMT3a and DNMT3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Okano, M., Xie, S. & Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nature Genet. 19, 219–220 (1998).
Chen, T., Tsujimoto, N. & Li, E. The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol. Cell. Biol. 24, 9048–9058 (2004).
Ofir, R., Wong, A. C., McDermid, H. E., Skorecki, K. L. & Selig, S. Position effect of human telomeric repeats on replication timing. Proc. Natl Acad. Sci. USA 96, 11434–11439 (1999).
Jiang, G. et al. Testing the position-effect variegation hypothesis for facioscapulohumeral muscular dystrophy by analysis of histone modification and gene expression in subtelomeric 4q. Hum. Mol. Genet. 12, 2909–2921 (2003).
Bayne, R. A. L. et al. Sandwiching of a gene within 12 kb of a functional telomere and alpha satellite does not result in silencing. Hum Mol Genet. 3, 539–546 (1994).
Wright, W. E., Tesmer, V. M., Liao, M. L., & Shay, J. W. Normal human telomeres are not late replicating. Exp. Cell Res. 251, 492–499 (1999).
Ancelin, K. et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22, 3474–3487 (2002).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Wang, R. C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004).
Dynek, J. N. & Smith, S. Resolution of sister telomere association is required for progression through mitosis. Science 304, 97–100 (2004).
Buck, S. W. & Shore, D. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 9, 370–384 (1995).
Wiley, E. A. & Zakian, V. A. Extra telomeres, but not internal tracts of telomeric DNA, reduce transcriptional repression at Saccharomyces telomeres. Genetics 139, 67–79 (1995).
Marcand, S., Brevet, V. & Gilson, E. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 18, 3509–3519 (1999).
Teixeira, M. T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117, 323–335 (2004). This work shows that budding yeast telomerase activity preferentially acts on the shortest telomeres.
Samper, E., Flores, J. M. & Blasco, M. A. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep. 2, 800–807 (2001).
Hemann, M. T., Strong, M. A., Hao, L. Y. & Greider, C. W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107, 67–77 (2001). References 131 and 132 show that mammalian telomerase activity preferentially acts on the shortest telomeres.
Xu, G. L. et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 402, 187–191 (1999).
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 23, 185–188 (1999).
Cawthon, R. M. et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395 (2003).
Valdes, A. M. et al. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664 (2005).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17312–17315 (2004).
Haigis, M. C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β-cells. Cell 126, 941–954 (2006).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Chua, K. F. et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2, 67–76 (2005).
Roth, W. et al. PIASy-deficient mice display modest defects in IFN and Wnt signaling. J. Immunol. 173, 6189–6199 (2004).
Nacerddine, K. et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell. 9, 769–779 (2005).
Marciniak, R. A. et al. A novel telomere structure in a human alternative lengthening of telomeres cell line. Cancer Res. 65, 2730–2737 (2005).
Acknowledgements
I am indebted to E. Gilson (Ecole Normale Supérieure de Lyon, France) for useful discussions and critical reading of the manuscript. M.A.B.'s laboratory is funded by the Spanish Ministry of Education and Culture (MCyT), by the Regional Government of Madrid, the European Union and the Josef Steiner Cancer Research Award 2003.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Dyskeratosis congenita
-
A condition that is characterized by bone marrow failure, genetic instability, elevated cancer risk and other abnormalities. Mutations in telomerase components have been described in some cases.
- Aplastic anaemia
-
A condition that results from a peripheral deficiency of all bone-marrow-derived haematopoietic lineages, such as red blood cells, platelets and leukocytes. There are many causes of this potentially fatal clinical syndrome. Mutations in telomerase components have been described in some cases.
- Heterochromatin
-
Chromosomal material that is tightly coiled and generally inactive in terms of gene expression.
- Nucleosome
-
The fundamental unit into which DNA and histones are packaged in eukaryotic cells. It is the basic structural subunit of chromatin and consists of ∼200 bp of DNA wrapped around an octamer of histone proteins.
- Histone modifications
-
Histones undergo post-translational modifications that alter their interaction with DNA and nuclear proteins. In particular, the tails of histones H3 and H4 can be covalently modified at several residues. Modifications of the tail include methylation, acetylation, phosphorylation and ubiquitination, and affect several biological processes, including gene expression, DNA repair and chromosome condensation.
- DNA methylation
-
DNA methylation occurs predominantly in repetitive genomic regions that contain CpG residues. DNA methylation represses transcription directly by inhibiting the binding of specific transcription factors, and indirectly by recruiting methyl-CpG-binding proteins and their associated repressive chromatin-remodelling activities.
- PML body
-
Subnuclear compartments that are defined by the presence of the PML (promyelocytic leukaemia) protein. They have been associated with diverse nuclear functions including transcription, DNA repair, viral defense, stress, cell-cycle regulation, proteolysis and apoptosis.
- Pericentric heterochromatin
-
The late-replicating, gene-sparse, transcriptionally inactive, condensed chromatin regions that are rich in repeated sequence and occur near the centromeres of chromosomes.
- Small interfering RNA
-
A non-coding RNA (∼22 nucleotides long) that is derived from the processing of long dsRNA during RNAi. siRNAs direct the destruction or translation repression of mRNA targets that they hybridize with.
- ICF
-
Immunodeficiency–centromeric instability–facial anomalies syndrome (ICF) is an extremely rare autosomal recessive disease that is characterized by profound immunodeficiency. Many ICF patients carry mutations of the DNMT3B gene, leading to DNA-methylation defects.
- Rett syndrome
-
An X-linked dominant neurological disorder that mainly affects girls and is one of the most common causes of mental retardation in females. Typical Rett syndrome is due to a mutation in the MECP2 gene (methyl-CpG-binding protein 2) resulting in decreased DNA methylation.
Rights and permissions
About this article
Cite this article
Blasco, M. The epigenetic regulation of mammalian telomeres. Nat Rev Genet 8, 299–309 (2007). https://doi.org/10.1038/nrg2047
Issue Date:
DOI: https://doi.org/10.1038/nrg2047
This article is cited by
-
Association between telomere length and neuropsychological function at 4–5 years in children from the INMA project: a cross-sectional study
European Child & Adolescent Psychiatry (2024)
-
Early Environment and Telomeres: a Long-Term Toxic Relationship
Current Environmental Health Reports (2023)
-
Sexual Dimorphism in Telomere Length in Childhood Autism
Journal of Autism and Developmental Disorders (2023)
-
Columnar structure of human telomeric chromatin
Nature (2022)
-
TGF-β controls stromal telomere length through epigenetic modifications
3 Biotech (2022)