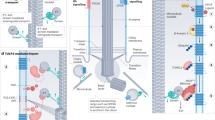
Key Points
-
Over the past 10 years, primary cilia have been found to be required for hedgehog (Hh) signalling during development and have been implicated in cystic kidney disease and complex inherited disorders called ciliopathies.
-
In early vertebrate embryos, the primary cilium seems to be dedicated to transduction of Hh signals. Proteins required for building cilia, including the anterograde kinesin-2 motor, components of the intraflagellar transport B (IFTB) complex and a subset of basal-body-associated proteins, are required for Hh signalling in mice and zebrafish, and core components of the Hh pathway localize to cilia in a ligand-dependent manner. By contrast, the evidence suggests that other developmental signalling pathways do not depend on cilia.
-
All Hh signalling in embryos, adult stem cells and tumours depends on the primary cilium.
-
Based on the phenotypes of mutants that lack different components of the IFT machinery, regulated trafficking within the cilium determines the level of Hh signalling.
-
The fused (FU) and KIF7 proteins provide links between IFT and cilia and the Hh pathway. FU and COS2 (the homologue of KIF7) are required for Hh signalling in Drosophila melanogaster, whereas in other invertebrates they are required for ciliogenesis. In vertebrates, FU and KIF7 function in both cilia formation and Hh signalling.
-
Planar cell polarity (PCP) pathways can control the position of primary cilia, and mammalian homologues of proteins that function as PCP effectors in D. melanogaster are required for ciliogenesis. These connections between PCP and cilia position may have a role in cilia function in the inner ear and in the kidney.
Abstract
The primary cilium has recently stepped into the spotlight, as a flood of data show that this organelle has crucial roles in vertebrate development and human genetic diseases. Cilia are required for the response to developmental signals, and evidence is accumulating that the primary cilium is specialized for hedgehog signal transduction. The formation of cilia, in turn, is regulated by other signalling pathways, possibly including the planar cell polarity pathway. The cilium therefore represents a nexus for signalling pathways during development. The connections between cilia and developmental signalling have begun to clarify the basis of human diseases associated with ciliary dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pedersen, L. B., Veland, I. R., Schroder, J. M. & Christensen, S. T. Assembly of primary cilia. Dev. Dyn. 237, 1993–2006 (2008).
Silverman, M. A. & Leroux, M. R. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 19, 306–316 (2009).
Lancaster, M. A. & Gleeson, J. G. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 19, 220–229 (2009).
Patel, V., Chowdhury, R. & Igarashi, P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 18, 99–106 (2009).
Zhou, J. Polycystins and primary cilia: primers for cell cycle progression. Annu. Rev. Physiol. 71, 83–113 (2009).
Harris, P. C. & Torres, V. E. Polycystic kidney disease. Annu. Rev. Med. 60, 321–337 (2009).
Gerdes, J., Davis, E. E. & Katsanis, N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45 (2009).
Badano, J. L., Mitsuma, N., Beales, P. L. & Katsanis, N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 (2006).
Baker, K. & Beales, P. L. Making sense of cilia in disease: the human ciliopathies. Am. J. Med. Genet. C Semin. Med. Genet. 151C, 281–295 (2009).
Tobin, J. L. & Beales, P. L. The nonmotile ciliopathies. Genet. Med. 11, 386–402 (2009).
Huangfu, D. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003).
Blacque, O. E. et al. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol. Biol. Cell 17, 5053–5062 (2006).
Cole, D. G. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic 4, 435–442 (2003).
Iomini, C., Babaev-Khaimov, V., Sassaroli, M. & Piperno, G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 153, 13–24 (2001).
Tran, P. et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nature Genet. 40, 403–410 (2008). This study provided the first phenotypic description of an IFTA mutant and showed that disrupting IFTA, in contrast to other IFT mutants, causes hyperactivation of the Hh pathway.
Kim, J. C. et al. The Bardet–Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nature Genet. 36, 462–470 (2004).
Nachury, M. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Huangfu, D. & Anderson, K. V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl Acad. Sci. USA 102, 11325–11330 (2005).
May, S. R. et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287, 378–389 (2005).
Ocbina, P. J. & Anderson, K. V. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn. 237, 2030–2038 (2008).
Liu, A., Wang, B. & Niswander, L. A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111 (2005).
Huang, P. & Schier, A. F. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136, 3089–3098 (2009). References 20 and 22 demonstrate a conserved requirement for cilia in Hh signalling, but not Wnt signalling, in vertebrates.
Ede, D. A. & Kelly, W. A. Developmental abnormalities in the trunk and limbs of the talpid3 mutant of the fowl. J. Embryol. Exp. Morphol. 12, 339–356 (1964).
Ede, D. A. & Kelly, W. A. Developmental abnormalities in the head region of the talpid mutant of the fowl. J. Embryol. Exp. Morphol. 12, 161–182 (1964).
Lewis, K. E. et al. Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development 126, 2397–2407 (1999).
Davey, M. G., James, J., Paton, I. R., Burt, D. W. & Tickle, C. Analysis of talpid3 and wild-type chicken embryos reveals roles for Hedgehog signalling in development of the limb bud vasculature. Dev. Biol. 301, 155–165 (2007).
Yin, Y. et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development 136, 655–664 (2009).
Delous, M. et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nature Genet. 39, 875–81 (2007).
Ferrante, M. et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nature Genet. 38, 112–117 (2006).
Ferrante, M. I. et al. Identification of the gene for oral-facial-digital type I syndrome. Am. J. Hum. Genet. 68, 569–576 (2001).
Vierkotten, J., Dildrop, R., Peters, T., Wang, B. & Ruther, U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 134, 2569–2577 (2007).
Weatherbee, S. D., Niswander, L. A. & Anderson, K. V. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum. Mol. Genet. 18, 4565–4575 (2009).
Ruiz-Perez, V. L. et al. Mutations in a new gene in Ellis–van Creveld syndrome and Weyers acrodental dysostosis. Nature Genet. 24, 283–286 (2000).
Ruiz-Perez, V. L. et al. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis–van Creveld syndrome. Am. J. Hum. Genet. 72, 728–732 (2003).
Rohatgi, R., Milenkovic, L. & Scott, M. Patched1 regulates Hedgehog signaling at the primary cilium. Science 317, 372–376 (2007).
Corbit, K. C. et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005).
Kim, J., Kato, M. & Beachy, P. A. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl Acad. Sci. USA 106, 21666–21671 (2009).
Ocbina, P. J., Tuson, M. & Anderson, K. V. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE 4, e6839 (2009).
Haycraft, C. et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, e53 (2005). References 35, 36 and 39 showed that components of the Hh pathway localize to cilia, providing cellular evidence of a direct connection between cilia and Hh signalling.
Endoh-Yamagami, S. et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr. Biol. 19, 1320–1326 (2009).
Jia, J. et al. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev. Biol. 330, 452–460 (2009).
Chen, M.-H. et al. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 23, 1910–1928 (2009).
Houde, C. et al. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev. Biol. 300, 523–533 (2006).
Iomini, C., Li, L., Esparza, J. M. & Dutcher, S. K. Retrograde IFT mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics 183, 885–896 (2009).
Cortellino, S. et al. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev. Biol. 325, 225–237 (2009).
Stottmann, R. W., Tran, P. V., Turbe-Doan, A. & Beier, D. R. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev. Biol. 339, 166–178 (2009).
Cole, D. G. et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 (1998).
Ou, G., Blacque, O. E., Snow, J. J., Leroux, M. R. & Scholey, J. M. Functional coordination of intraflagellar transport motors. Nature 436, 583–587 (2005).
Qin, H. et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15, 1695–1699 (2005).
Milenkovic, L., Scott, M. P. & Rohatgi, R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 187, 365–374 (2009).
Sisson, J. C., Ho, K. S., Suyama, K. & Scott, M. P. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90, 235–245 (1997).
Wang, G., Amanai, K., Wang, B. & Jiang, J. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev. 14, 2893–2905 (2000).
Aikin, R. A., Ayers, K. L. & Therond, P. P. The role of kinases in the Hedgehog signalling pathway. EMBO Rep. 9, 330–336 (2008).
Farzan, S. F. et al. Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Curr. Biol. 18, 1215–1220 (2008).
Liem, K. F. Jr, He, M., Ocbina, P. J. & Anderson, K. V. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc. Natl Acad. Sci. USA 106, 13377–13382 (2009).
Cheung, H. O. et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci. Signal. 2, ra29 (2009).
Tay, S. Y., Ingham, P. W. & Roy, S. A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development 132, 625–634 (2005). References 55–57 together with reference 40 show that the COS2 homologue KIF7 is required for Hh signalling in vertebrate development, which is in contrast with previous reports that indicated its role in Hh signalling was not evolutionarily conserved.
Wolff, C., Roy, S. & Ingham, P. W. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr. Biol. 13, 1169–1181 (2003).
Chen, M. H., Gao, N., Kawakami, T. & Chuang, P. T. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol. Cell. Biol. 25, 7042–7053 (2005).
Merchant, M. et al. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol. Cell. Biol. 25, 7054–7068 (2005).
Wilson, C. W. et al. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature 459, 98–102 (2009). This paper establishes FU as a link between the Hh pathway and cilia in vertebrates, demonstrating that it is required both for cilia structure and Hh signalling in zebrafish, and for motile cilia formation in mammals, although the link with Hh seems to have been lost in mammals.
Evangelista, M. et al. Kinome siRNA screen identifies regulators of ciliogenesis and hedgehog signal transduction. Sci. Signal. 1, ra7 (2008).
Varjosalo, M. et al. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell 133, 537–548 (2008). References 36 and 63 showed that KIF7–GFP fusion proteins localize to cilia, and reference 63 further demonstrated that this localization is ligand-dependent. The authors proposed that KIF7 may act as an accessory anterograde motor linking cilia trafficking with the Hh pathway.
Glazer, A. M. et al. The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev. Biol. 337, 148–156 (2009).
Rink, J. C., Gurley, K. A., Elliott, S. A. & Sánchez Alvarado, A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326, 1406–1410 (2009). References 64 and 65 show that several components of the Hh pathway in D. melanogaster — Iguana, FU and COS2 — are required in a separate invertebrate lineage for cilia formation. This provided evidence that the connection between the Hh pathway and cilia might be evolutionarily ancient.
Ross, A. J. et al. Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nature Genet. 37, 1135–1140 (2005).
Gerdes, J. et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature Genet. 39, 1350–1360 (2007).
Simons, M. et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genet. 37, 537–543 (2005).
Corbit, K. et al. Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nature Cell Biol. 10, 70–76 (2008).
Bergmann, C. et al. Loss of Nephrocystin-3 function can cause embryonic lethality, Meckel–Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am. J. Hum. Genet. 82, 959–970 (2008).
Jonassen, J. A., San Agustin, J., Follit, J. A. & Pazour, G. J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 183, 377–384 (2008).
Wiens, C. J. et al. The Bardet–Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signalling. J. Biol. Chem. 5 Mar 2010 (doi:10.1074/jbc.M109.070953).
Barrow, J. et al. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev. Biol. 312, 312–320 (2007).
Chazaud, C. & Rossant, J. Disruption of early proximodistal patterning and AVE formation in Apc mutants. Development 133, 3379–3387 (2006).
Zeng, L. et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90, 181–192 (1997).
Mukhopadhyay, M. et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell 1, 423–434 (2001).
Ishikawa, T. O., Tamai, Y., Li, Q., Oshima, M. & Taketo, M. M. Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev. Biol. 253, 230–246 (2003).
Greene, N. D., Gerrelli, D., Van Straaten, H. W. & Copp, A. J. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech. Dev. 73, 59–72 (1998).
Curtin, J. A. et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 13, 1129–1133 (2003).
Schneider, L. et al. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866 (2005).
Schneider, L. et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol. Biochem. 25, 279–292.
Danilov, A. I. et al. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia 57, 136–152 (2009).
Klinghoffer, R. A., Hamilton, T. G., Hoch, R. & Soriano, P. An allelic series at the PDGFαR locus indicates unequal contributions of distinct signaling pathways during development. Dev. Cell 2, 103–113 (2002).
Soriano, P. The PDGFα receptor is required for neural crest cell development and for normal patterning of the somites. Development 124, 2691–2700 (1997).
Ruiz i Altaba, A., Palma, V. & Dahmane, N. Hedgehog–Gli signalling and the growth of the brain. Nature Rev. Neurosci. 3, 24–33 (2002).
Chizhikov, V. et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J. Neurosci. 27, 9780–9789 (2007).
Vaillant, C. & Monard, D. SHH pathway and cerebellar development. Cerebellum 8, 291–301 (2009).
Han, Y. et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nature Neurosci. 11, 277–284 (2008).
Lee, J. D. & Anderson, K. V. Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev. Dyn. 237, 3464–3476 (2008).
Hong, S. K. & Dawid, I. B. FGF-dependent left-right asymmetry patterning in zebrafish is mediated by Ier2 and Fibp1. Proc. Natl Acad. Sci. USA 106, 2230–2235 (2009).
Neugebauer, J. M., Amack, J. D., Peterson, A. G., Bisgrove, B. W. & Yost, H. J. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458, 651–654 (2009).
Yamauchi, H., Miyakawa, N., Miyake, A. & Itoh, N. Fgf4 is required for left-right patterning of visceral organs in zebrafish. Dev. Biol. 332, 177–185 (2009).
Sarmah, B., Winfrey, V. P., Olson, G. E., Appel, B. & Wente, S. R. A role for the inositol kinase Ipk1 in ciliary beating and length maintenance. Proc. Natl Acad. Sci. USA 104, 19843–19848 (2007).
Bielas, S. L. et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nature Genet. 41, 1032–1036 (2009).
Wang, Y., Guo, N. & Nathans, J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147–2156 (2006).
Montcouquiol, M. et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173–177 (2003).
Wang, J. et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nature Genet. 37, 980–985 (2005).
Qian, D. et al. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121–133 (2007).
Jones, C. et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nature Genet. 40, 69–77 (2008). This study revealed a complex link between cilia and the PCP pathway, wherein PCP is required for the polarized orientation of the hair cell primary cilium, which in turn acts in parallel with the PCP pathway to promote correct orientation of stereocilia in the cochlea.
Montcouquiol, M. et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265–5275 (2006).
Park, T., Mitchell, B., Abitua, P., Kintner, C. & Wallingford, J. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nature Genet. 40, 871–879 (2008).
Park, T. J., Haigo, S. L. & Wallingford, J. B. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nature Genet. 38, 303–311 (2006).
Gray, R. S. et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nature Cell Biol. 11, 1225–1232 (2009).
Heydeck, W., Zeng, H. & Liu, A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev. Dyn. 238, 3035–3042 (2009).
Zeng, H., Hoover, A. N. & Liu, A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev. Biol. 339, 418–428 (2010). References 101–105 show that components of the PCP pathway can function upstream of cilia in certain contexts by regulating the apical docking of basal bodies in conjunction with vesical trafficking components.
Hahn, H. et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nature Med. 4, 619–622 (1998).
Daya-Grosjean, L. & Couve-Privat, S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 225, 181–192 (2005).
Marino, S. Medulloblastoma: developmental mechanisms out of control. Trends Mol. Med. 11, 17–22 (2005).
Teglund, S. & Toftgard, R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim. Biophys. Acta 1805, 181–208 (2010).
Theunissen, J. W. & de Sauvage, F. J. Paracrine Hedgehog signaling in cancer. Cancer Res. 69, 6007–6010 (2009).
Han, Y. G. et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nature Med. 15, 1062–1065 (2009).
Wong, S. Y. et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nature Med. 15, 1055–1061 (2009). References 111 and 112 show that cilia are important for the modulation of Hh signalling in two types of tumour, that ablation of cilia in cells with activated SMO suppresses tumour formation and that activation of GLI2 results in tumours only when cilia are absent. These results emphasize that cilia have both positive and negative roles in Hh signalling.
Nishio, S. et al. Loss of oriented cell division does not initiate cyst formation. J. Am. Soc. Nephrol. 21, 295–302 (2009).
Gill, P. S. & Rosenblum, N. D. Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle 5, 1426–1430 (2006).
Hu, M. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133, 569–578 (2006).
Yu, J., Carroll, T. J. & McMahon, A. P. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301–5312 (2002).
Benzing, T., Simons, M. & Walz, G. Wnt signaling in polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1389–1398 (2007).
Phillips, C. L. et al. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J. Am. Soc. Nephrol. 15, 1744–1755 (2004).
Shiba, D. et al. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J. Cell Sci. 122, 44–54 (2009).
Otto, E. A. et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nature Genet. 34, 413–420 (2003).
Lin, F. et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc. Natl Acad. Sci. USA 100, 5286–5291 (2003).
Saadi-Kheddouci, S. et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the β-catenin gene. Oncogene 20, 5972–5981 (2001).
Lancaster, M. A. et al. Impaired Wnt–β-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nature Med. 15, 1046–1054 (2009).
Fischer, E. et al. Defective planar cell polarity in polycystic kidney disease. Nature Genet. 38, 21–23 (2006).
Saburi, S. et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nature Genet. 40, 1010–1015 (2008).
Casal, J., Lawrence, P. A. & Struhl, G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133, 4561–4572 (2006).
Berbari, N. F., Lewis, J. S., Bishop, G. A., Askwith, C. C. & Mykytyn, K. Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl Acad. Sci. USA 105, 4242–4246 (2008).
Toriello, H. V. & Parisi, M. A. Cilia and the ciliopathies: an introduction. Am. J. Med. Genet. C Semin. Med. Genet. 151C, 261–262 (2009).
Ruiz-Perez, V. L. et al. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development 134, 2903–2912 (2007).
Brancati, F. et al. MKS3/TMEM67 mutations are a major cause of COACH syndrome, a Joubert syndrome related disorder with liver involvement. Hum. Mutat. 30, E432–E442 (2009).
Kulaga, H. et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nature Genet. 36, 994–998 (2004).
Mykytyn, K. et al. Bardet–Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc. Natl Acad. Sci. USA 101, 8664–8669 (2004).
Nishimura, D. Y. et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc. Natl Acad. Sci. USA 101, 16588–16593 (2004).
Lee, J. H. & Gleeson, J. G. The role of primary cilia in neuronal function. Neurobiol. Dis. 22 Jan 2010 (doi:10.1016/j.nbd.2009.12.022).
Davenport, J. et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586–1594 (2007).
Omori, Y. et al. elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nature Cell Biol. 10, 437–444 (2008).
Caspary, T., Larkins, C. E. & Anderson, K. V. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell 12, 767–778 (2007).
Jia, J., Tong, C. & Jiang, J. Smoothened transduces Hedgehog signal by physically interacting with Costal2/Fused complex through its C-terminal tail. Genes Dev. 17, 2709–2720 (2003).
Lefers, M. A., Wang, Q. T. & Holmgren, R. A. Genetic dissection of the Drosophila Cubitus interruptus signaling complex. Dev. Biol. 236, 411–420 (2001).
Lum, L. et al. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol. Cell 12, 1261–1274 (2003).
Osterlund, T. & Kogerman, P. Hedgehog signalling: how to get from Smo to Ci and Gli. Trends Cell Biol. 16, 176–180 (2006).
Pan, J., You, Y., Huang, T. & Brody, S. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J. Cell Sci. 120, 1868–1876 (2007).
Cantagrel, V. et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 83, 170–179 (2008).
Mykytyn, K. et al. Evaluation of complex inheritance involving the most common Bardet–Biedl syndrome locus (BBS1). Am. J. Hum. Genet. 72, 429–437 (2003).
Nishimura, D. Y. et al. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2). Hum. Mol. Genet. 10, 865–874 (2001).
Chiang, A. P. et al. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3). Am. J. Hum. Genet. 75, 475–484 (2004).
Mykytyn, K. et al. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nature Genet. 28, 188–191 (2001).
Li, J. B. et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117, 541–552 (2004).
Badano, J. L. et al. Identification of a novel Bardet–Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am. J. Hum. Genet. 72, 650–658 (2003).
Ansley, S. J. et al. Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425, 628–633 (2003).
Nishimura, D. Y. et al. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet–Biedl syndrome gene. Am. J. Hum. Genet. 77, 1021–1033 (2005).
Stoetzel, C. et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nature Genet. 38, 521–524 (2006).
Kudryashova, E., Wu, J., Havton, L. A. & Spencer, M. J. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Hum. Mol. Genet. 18, 1353–1367 (2009).
Chiang, A. P. et al. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet–Biedl syndrome gene (BBS11). Proc. Natl Acad. Sci. USA 103, 6287–6292 (2006).
Cossee, M. et al. Use of SNP array analysis to identify a novel TRIM32 mutation in limb-girdle muscular dystrophy type 2H. Neuromuscul. Disord. 19, 255–260 (2009).
Stoetzel, C. et al. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet–Biedl syndrome. Am. J. Hum. Genet. 80, 1–11 (2007).
Dagoneau, N. et al. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am. J. Hum. Genet. 84, 706–711 (2009).
Arts, H. H. et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nature Genet. 39, 882–888 (2007).
Beales, P. L. et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nature Genet. 39, 727–729 (2007).
Murcia, N. S. et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127, 2347–2355 (2000).
Marszalek, J. R., Ruiz-Lozano, P., Roberts, E., Chien, K. R. & Goldstein, L. S. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl Acad. Sci. USA 96, 5043–5048 (1999).
Nonaka, S. et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998).
Kyttälä, M. et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nature Genet. 38, 155–157 (2006).
Izraeli, S. et al. Genetic evidence that Sil is required for the Sonic Hedgehog response pathway. Genesis 31, 72–77 (2001).
Kumar, A., Girimaji, S. C., Duvvari, M. R. & Blanton, S. H. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am. J. Hum. Genet. 84, 286–290 (2009).
Singla, V., Romaguera-Ros, M., Garcia-Verdugo, J. M. & Reiter, J. F. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell 18, 410–424 (2010).
Acknowledgements
This work was funded by US National Institutes of Health grant NS044385 (to K.V.A.). S.C.G. is an American Cancer Society postdoctoral fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Ciliopathies
-
Human disorders affecting diverse organ systems in which the underlying cellular defect has been found to be structural or functional abnormalities of cilia.
- Basal bodies
-
Cylindrical, microtubule-based structures at the base of cilia from which ciliary axonemes are nucleated. They are derived from mother centrioles.
- Axoneme
-
The long projection of the cilium into the extracellular space. It is composed of a circular array of nine microtubule doublets.
- Anterograde trafficking
-
Transport towards the microtubule plus end (the cilia tip).
- Retrograde trafficking
-
Transport towards the microtubule minus end (the cilia base).
- Mother centriole
-
Centrioles are tube-shaped structures composed of nine triplets of microtubules. One of the two centrioles is the mother centriole, which forms the basal body.
- Polydactyly
-
The formation of additional digits on the limbs. Additional posterior digits are referred to as postaxial, and additional anterior digits are referred to as preaxial.
- Pericentriolar material
-
A network of fibres and associated proteins that surround the centriole and contain the microtubule-organizing activity of the centrosome.
- Hydrocephalus
-
The build-up of cerebrospinal fluid within the ventricles of the brain.
- Kinome
-
The set of protein kinases in the genome of a given organism.
- Metazoan
-
Multicellular organisms that, with the exception of sponges, have specialized cell types. Historically referred to as the kingdom 'Animalia'.
- Convergent extension
-
A morphogenetic movement characterized by the intercalation and elongation of cells, which causes a structure to generally become longer and thinner.
- Subventricular zone
-
A layer of cells lining the ventricles of the brain in which neurogenesis takes place in adult mammals.
- Kupffer's vesicle
-
A ciliated organ of the fish embryo that serves to generate asymmetry during development, functioning analogously to the mammalian node.
- Hair cells
-
The sensory cells in the vertebrate auditory system. They are contained within the cochlea.
- Organ of Corti
-
The organ in the mammalian inner ear that contains the hair cells.
- Cochlea
-
The portion of the inner ear that contains the sensory organs of hearing.
- Kinocilium
-
The specialized primary cilium of the hair cells of the cochlea.
- Stereocilia
-
The actin-based sensory organelles that form a polarized chevron-shaped structure in the hair cell.
Rights and permissions
About this article
Cite this article
Goetz, S., Anderson, K. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11, 331–344 (2010). https://doi.org/10.1038/nrg2774
Issue Date:
DOI: https://doi.org/10.1038/nrg2774
This article is cited by
-
Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease
Cellular & Molecular Biology Letters (2024)
-
Deletion of Aurora kinase A prevents the development of polycystic kidney disease in mice
Nature Communications (2024)
-
The peroxisome: an update on mysteries 3.0
Histochemistry and Cell Biology (2024)
-
In vitro-generated human muscle reserve cells are heterogeneous for Pax7 with distinct molecular states and metabolic profiles
Stem Cell Research & Therapy (2023)
-
IK is essentially involved in ciliogenesis as an upstream regulator of oral-facial-digital syndrome ciliopathy gene, ofd1
Cell & Bioscience (2023)