Key Points
-
Expression of target genes in response to extracellular stimuli is activated by means that include activation of RNA polymerase II (Pol II)-dependent transcription.
-
Multiple processes during the early stages of Pol II-dependent transcription are subject to regulation and can therefore function as the rate-limiting step during activation of target genes.
-
Activator-dependent recruitment of Pol II and the general transcription factors is essential for high levels of transcription at inducible genes. This process must occur at all induced genes before activation, whether or not recruitment of polymerase is rate-limiting with regards to activation.
-
The kinetics of Pol II recruitment and transcription initiation can be stimulated by nucleosome-remodelling complexes that use the energy from ATP hydrolysis to move or displace histones from DNA.
-
Co-activators are essential for activator-dependent recruitment of Pol II and the general transcription factors. Mediator and the histone acetyltransferase complex SAGA are well-characterized examples of co-activators.
-
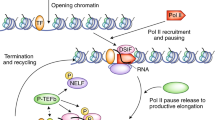
Transcription can be regulated at steps that occur after the recruitment of Pol II. These steps include the release of Pol II from a paused state close to the promoter into active transcription elongation in the coding region of the gene.
-
The rate-limiting step of transcription can differ between various inducible genes.
-
Signalling kinases such as MAPKs sometimes localize to the promoters of target genes at which they can function as transcriptional activators, rapidly facilitating the switch between activated and repressed states of gene expression.
Abstract
The rapid activation of gene expression in response to stimuli occurs largely through the regulation of RNA polymerase II-dependent transcription. In this Review, we discuss events that occur during the transcription cycle in eukaryotes that are important for the rapid and specific activation of gene expression in response to external stimuli. In addition to regulated recruitment of the transcription machinery to the promoter, it has now been shown that control steps can include chromatin remodelling and the release of paused polymerase. Recent work suggests that some components of signal transduction cascades also play an integral part in activating transcription at target genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zeitlinger, J. et al. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nature Genet. 39, 1512–1516 (2007).
Muse, G. W. et al. RNA polymerase is poised for activation across the genome. Nature Genet. 39, 1507–1511 (2007).
Kim, T. H. et al. A high-resolution map of active promoters in the human genome. Nature 436, 876–880 (2005).
Guenther, M. G., Levine, S. S., Boyer, L. A., Jaenisch, R. & Young, R. A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Lee, T. I. et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
de Nadal, E. & Posas, F. Multilayered control of gene expression by stress-activated protein kinases. EMBO J. 29, 4–13 (2010).
Fuda, N. J., Ardehali, M. B. & Lis, J. T. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461, 186–192 (2009).
Roeder, R. G. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 579, 909–915 (2005).
Ptashne, M. & Gann, A. Transcriptional activation by recruitment. Nature 386, 569–577 (1997).
Orphanides, G. & Reinberg, D. A unified theory of gene expression. Cell 108, 439–451 (2002). A comprehensive review of all aspects of gene expression, including those that are not described in this Review, such as transcription elongation, pre-mRNA processing and export and protein translation.
Traven, A., Jelicic, B. & Sopta, M. Yeast Gal4: a transcriptional paradigm revisited. EMBO Rep. 7, 496–499 (2006).
Brent, R. & Ptashne, M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell 43, 729–736 (1985).
Wu, Y., Reece, R. J. & Ptashne, M. Quantitation of putative activator–target affinities predicts transcriptional activating potentials. EMBO J. 15, 3951–3963 (1996).
Carrozza, M. J., John, S., Sil, A. K., Hopper, J. E. & Workman, J. L. Gal80 confers specificity on HAT complex interactions with activators. J. Biol. Chem. 277, 24648–24652 (2002).
Blank, T. E., Woods, M. P., Lebo, C. M., Xin, P. & Hopper, J. E. Novel Gal3 proteins showing altered Gal80p binding cause constitutive transcription of Gal4p-activated genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 2566–2575 (1997).
Yano, K. & Fukasawa, T. Galactose-dependent reversible interaction of Gal3p with Gal80p in the induction pathway of Gal4p-activated genes of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 94, 1721–1726 (1997).
Platt, A. & Reece, R. J. The yeast galactose genetic switch is mediated by the formation of a Gal4p–Gal80p–Gal3p complex. EMBO J. 17, 4086–4091 (1998).
Suzuki-Fujimoto, T. et al. Analysis of the galactose signal transduction pathway in Saccharomyces cerevisiae: interaction between Gal3p and Gal80p. Mol. Cell. Biol. 16, 2504–2508 (1996).
Zenke, F. T. et al. Activation of Gal4p by galactose-dependent interaction of galactokinase and Gal80p. Science 272, 1662–1665 (1996).
Jiang, F., Frey, B. R., Evans, M. L., Friel, J. C. & Hopper, J. E. Gene activation by dissociation of an inhibitor from a transcriptional activation domain. Mol. Cell. Biol. 29, 5604–5610 (2009).
Bhaumik, S. R. & Green, M. R. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 15, 1935–1945 (2001).
Bryant, G. O. & Ptashne, M. Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell 11, 1301–1309 (2003).
Larschan, E. & Winston, F. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15, 1946–1956 (2001).
Kuras, L., Borggrefe, T. & Kornberg, R. D. Association of the Mediator complex with enhancers of active genes. Proc. Natl Acad. Sci. USA 100, 13887–13891 (2003).
Ansari, A. Z. et al. Transcriptional activating regions target a cyclin-dependent kinase. Proc. Natl Acad. Sci. USA 99, 14706–14709 (2002).
Koh, S. S., Ansari, A. Z., Ptashne, M. & Young, R. A. An activator target in the RNA polymerase II holoenzyme. Mol. Cell 1, 895–904 (1998).
Lemieux, K. & Gaudreau, L. Targeting of Swi/Snf to the yeast GAL1 UAS G requires the Mediator, TAFIIs, and RNA polymerase II. EMBO J. 23, 4040–4050 (2004).
Bhaumik, S. R. & Green, M. R. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22, 7365–7371 (2002).
Larschan, E. & Winston, F. The Saccharomyces cerevisiae Srb8–Srb11 complex functions with the SAGA complex during Gal4-activated transcription. Mol. Cell Biol. 25, 114–123 (2005).
Fischer, J. A., Giniger, E., Maniatis, T. & Ptashne, M. GAL4 activates transcription in Drosophila. Nature 332, 853–856 (1988).
Kakidani, H. & Ptashne, M. GAL4 activates gene expression in mammalian cells. Cell 52, 161–167 (1988).
De Val, S. et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 135, 1053–1064 (2008).
Rhodius, V. A. & Busby, S. J. Positive activation of gene expression. Curr. Opin. Microbiol. 1, 152–159 (1998).
Campos, E. I. & Reinberg, D. Histones: annotating chromatin. Annu. Rev. Genet. 43, 559–599 (2009).
Archer, T. K., Cordingley, M. G., Wolford, R. G. & Hager, G. L. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Mol. Cell. Biol. 11, 688–698 (1991).
Workman, J. L. & Roeder, R. G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell 51, 613–622 (1987).
Lorch, Y., LaPointe, J. W. & Kornberg, R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 49, 203–210 (1987).
Knezetic, J. A. & Luse, D. S. The presence of nucleosomes on a DNA template prevents initiation by RNA polymerase II in vitro. Cell 45, 95–104 (1986).
Cairns, B. R. The logic of chromatin architecture and remodelling at promoters. Nature 461, 193–198 (2009).
Grant, P. A. et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11, 1640–1650 (1997).
Steger, D. J., Eberharter, A., John, S., Grant, P. A. & Workman, J. L. Purified histone acetyltransferase complexes stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc. Natl Acad. Sci. USA 95, 12924–12929 (1998).
Ikeda, K., Steger, D. J., Eberharter, A. & Workman, J. L. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol. Cell. Biol. 19, 855–863 (1999).
Casamassimi, A. & Napoli, C. Mediator complexes and eukaryotic transcription regulation: an overview. Biochimie 89, 1439–1446 (2007).
Kimbrel, E. A. et al. Systematic in vivo structure-function analysis of p300 in hematopoiesis. Blood 114, 4804–4812 (2009).
Jiang, H. et al. PCAF interacts with Tax and stimulates Tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19, 8136–8145 (1999).
Qiu, H. et al. An array of coactivators is required for optimal recruitment of TATA binding protein and RNA polymerase II by promoter-bound Gcn4p. Mol. Cell. Biol. 24, 4104–4117 (2004).
Clapier, C. R. & Cairns, B. R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304 (2009).
Bryant, G. O. et al. Activator control of nucleosome occupancy in activation and repression of transcription. PLoS Biol. 6, 2928–2939 (2008). The authors show that there are two mechanisms of nucleosome loss from the Gal genes following exposure to galactose, one of which is dependent on activator-mediated recruitment of the nucleosome-remodelling complex SWI/SNF and a slower pathway that is independent of SWI/SNF. They also confirm that repression can occur at Gal genes in both the presence and absence of nucleosomes, showing that nucleosomes are not essential for repression at inducible promoters.
Yudkovsky, N., Logie, C., Hahn, S. & Peterson, C. L. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13, 2369–2374 (1999).
Soutoglou, E. & Talianidis, I. Coordination of PIC assembly and chromatin remodeling during differentiation-induced gene activation. Science 295, 1901–1904 (2002).
Hassan, A. H., Neely, K. E. & Workman, J. L. Histone acetyltransferase complexes stabilize SWI/SNF binding to promoter nucleosomes. Cell 104, 817–827 (2001).
Hassan, A. H. et al. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell 111, 369–379 (2002).
Chandy, M., Gutierrez, J. L., Prochasson, P. & Workman, J. L. SWI/SNF displaces SAGA-acetylated nucleosomes. Eukaryot. Cell 5, 1738–1747 (2006).
Carey, M., Li, B. & Workman, J. L. RSC exploits histone acetylation to abrogate the nucleosomal block to RNA polymerase II elongation. Mol. Cell 24, 481–487 (2006).
Reinke, H. & Horz, W. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell 11, 1599–1607 (2003).
Steger, D. J., Haswell, E. S., Miller, A. L., Wente, S. R. & O'Shea, E. K. Regulation of chromatin remodeling by inositol polyphosphates. Science 299, 114–116 (2003).
Sharma, V. M., Li, B. & Reese, J. C. SWI/SNF-dependent chromatin remodeling of RNR3 requires TAFIIs and the general transcription machinery. Genes Dev. 17, 502–515 (2003).
He, Q., Battistella, L. & Morse, R. H. Mediator requirement downstream of chromatin remodeling during transcriptional activation of CHA1 in yeast. J. Biol. Chem. 283, 5276–5286 (2008).
Zhang, H. & Reese, J. C. Exposing the core promoter is sufficient to activate transcription and alter coactivator requirement at RNR3. Proc. Natl Acad. Sci. USA 104, 8833–8838 (2007).
Adkins, M. W. & Tyler, J. K. Transcriptional activators are dispensable for transcription in the absence of Spt6-mediated chromatin reassembly of promoter regions. Mol. Cell 21, 405–416 (2006).
Schwabish, M. A. & Struhl, K. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 24, 10111–10117 (2004).
Lee, C. K., Shibata, Y., Rao, B., Strahl, B. D. & Lieb, J. D. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nature Genet. 36, 900–905 (2004).
Petesch, S. J. & Lis, J. T. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell 134, 74–84 (2008). This D. melanogaster study shows that heat shock induces a rapid loss of nucleosomes across the entire Hsp70 locus, and that this nucleosome loss occurs before and is independent of transcription. This is a key finding because it indicates that, although nucleosome disruption is required for optimal levels of Hsp70 expression, nucleosome loss on its own is not sufficient to activate transcription.
Wu, C., Wong, Y. C. & Elgin, S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell 16, 807–814 (1979).
Wu, C. The 5′ ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature 286, 854–860 (1980).
Lis, J. T. Imaging Drosophila gene activation and polymerase pausing in vivo. Nature 450, 198–202 (2007).
Winegarden, N. A., Wong, K. S., Sopta, M. & Westwood, J. T. Sodium salicylate decreases intracellular ATP, induces both heat shock factor binding and chromosomal puffing, but does not induce hsp 70 gene transcription in Drosophila. J. Biol. Chem. 271, 26971–26980 (1996).
Han, M. & Grunstein, M. Nucleosome loss activates yeast downstream promoters in vivo. Cell 55, 1137–1145 (1988). One of the first studies to show that nucleosome loss could activate transcription in vivo . Using a genetic approach, the authors inactivated histone H4 and found that the promoters of several genes were activated and transcription was initiated independently of the presence of transcriptional activators.
Wang, L., Liu, L. & Berger, S. L. Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 12, 640–653 (1998).
Kao, C. F. et al. Rad6 plays a role in transcriptional activation through ubiquitylation of histone H2B. Genes Dev. 18, 184–195 (2004).
Kim, J. et al. RAD6-mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell 137, 459–471 (2009).
Hwang, W. W. et al. A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol. Cell 11, 261–266 (2003).
Wood, A., Schneider, J., Dover, J., Johnston, M. & Shilatifard, A. The Paf1 complex is essential for histone monoubiquitination by the Rad6–Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J. Biol. Chem. 278, 34739–34742 (2003).
Robzyk, K., Recht, J. & Osley, M. A. Rad6-dependent ubiquitination of histone H2B in yeast. Science 287, 501–504 (2000).
Wood, A. et al. Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol. Cell 11, 267–274 (2003).
Ng, H. H., Dole, S. & Struhl, K. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J. Biol. Chem. 278, 33625–33628 (2003).
Xiao, T. et al. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol. Cell. Biol. 25, 637–651 (2005).
Weake, V. M. & Workman, J. L. Histone ubiquitination: triggering gene activity. Mol. Cell 29, 653–663 (2008).
Henry, K. W. et al. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 17, 2648–2663 (2003).
Daniel, J. A. et al. Deubiquitination of histone H2B by a yeast acetyltransferase complex regulates transcription. J. Biol. Chem. 279, 1867–1871 (2004).
Wyce, A. et al. H2B ubiquitylation acts as a barrier to Ctk1 nucleosomal recruitment prior to removal by Ubp8 within a SAGA-related complex. Mol. Cell 27, 275–288 (2007).
Saunders, A., Core, L. J. & Lis, J. T. Breaking barriers to transcription elongation. Nature Rev. Mol. Cell. Biol. 7, 557–567 (2006).
Rougvie, A. E. & Lis, J. T. The RNA polymerase II molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell 54, 795–804 (1988).
Lis, J. T., Mason, P., Peng, J., Price, D. H. & Werner, J. P-TEFb kinase recruitment and function at heat shock loci. Genes Dev. 14, 792–803 (2000).
Renner, D. B., Yamaguchi, Y., Wada, T., Handa, H. & Price, D. H. A highly purified RNA polymerase II elongation control system. J. Biol. Chem. 276, 42601–42609 (2001).
Law, A., Hirayoshi, K., O'Brien, T. & Lis, J. T. Direct cloning of DNA that interacts in vivo with a specific protein: application to RNA polymerase II and sites of pausing in Drosophila. Nucleic Acids Res. 26, 919–924 (1998).
Boettiger, A. N. & Levine, M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science 325, 471–473 (2009). This paper provides important clues into the possible reason for the presence of paused Pol II on the promoters of developmental control genes. Using in situ hybridizations, the authors found that genes with paused Pol II are activated more synchronously in different cells of the same tissue compared with genes at which the rate-limiting step in transcription occurs at the level of activator-dependent recruitment.
Lee, C. et al. NELF and GAGA factor are linked to promoter-proximal pausing at many genes in Drosophila. Mol. Cell. Biol. 28, 3290–3300 (2008).
Nechaev, S. et al. Global analysis of short RNAs reveals widespread promoter-proximal stalling and arrest of Pol II in Drosophila. Science 327, 335–338 (2010). This recent study found that short RNAs derived from stalled or paused Pol II are associated with approximately one-third of all D. melanogaster genes. These findings suggest that polymerase pausing might be a general feature of the early stages in the transcription cycle, even if it is not necessarily rate-limiting for all genes.
de Nadal, E., Casadome, L. & Posas, F. Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell. Biol. 23, 229–237 (2003).
Proft, M. et al. Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J. 20, 1123–1133 (2001).
Alepuz, P. M., de Nadal, E., Zapater, M., Ammerer, G. & Posas, F. Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 22, 2433–2442 (2003). This study showed that, surprisingly, phosphorylation of the target substrate of the Hog1 MAPK in yeast is not required for transcription activation at some genes. Instead recruitment of the MAPK itself is the key step required to activate transcription because artificial tethering of the MAPK to the promoter is sufficient to mediate stress-responsive activation at these genes.
Alepuz, P. M., Jovanovic, A., Reiser, V. & Ammerer, G. Stress-induced MAP kinase Hog1 is part of transcription activation complexes. Mol. Cell 7, 767–777 (2001).
Vicent, G. P. et al. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol. Cell 24, 367–381 (2006).
Zhang, H. M. et al. Mitogen-induced recruitment of ERK and MSK to SRE promoter complexes by ternary complex factor Elk-1. Nucleic Acids Res. 36, 2594–2607 (2008).
Hu, S. et al. Profiling the human protein–DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 139, 610–622 (2009).
Proft, M. et al. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol. Cell 23, 241–250 (2006).
Pascual-Ahuir, A., Struhl, K. & Proft, M. Genome-wide location analysis of the stress-activated MAP kinase Hog1 in yeast. Methods 40, 272–278 (2006).
Pokholok, D. K., Zeitlinger, J., Hannett, N. M., Reynolds, D. B. & Young, R. A. Activated signal transduction kinases frequently occupy target genes. Science 313, 533–536 (2006).
Mas, G. et al. Recruitment of a chromatin remodelling complex by the Hog1 MAP kinase to stress genes. EMBO J. 28, 326–336 (2009).
Lawrence, M. C. et al. Multiple chromatin-bound protein kinases assemble factors that regulate insulin gene transcription. Proc. Natl Acad. Sci. USA 106, 22181–22186 (2009). This paper shows that multiple MAPKs bind to the promoter of the insulin gene in human pancreatic β -cells in the presence of the proinflammatory cytokine IL-1 β . MAPKs at promoters of target genes might facilitate rapid switches in gene activation statusbecause these kinases promote activation or repression of insulin transcription depending on the glucose levels in the cellular environment.
D'Alessio, J. A., Wright, K. J. & Tjian, R. Shifting players and paradigms in cell-specific transcription. Mol. Cell 36, 924–931 (2009).
Egloff, S. & Murphy, S. Cracking the RNA polymerase II CTD code. Trends Genet. 24, 280–288 (2008).
Zimarino, V. & Wu, C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. Nature 327, 727–730 (1987).
Westwood, J. T., Clos, J. & Wu, C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature 353, 822–827 (1991).
Tulin, A. & Spradling, A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science 299, 560–562 (2003).
Tsukiyama, T., Becker, P. B. & Wu, C. ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature 367, 525–532 (1994).
Wang, Y. V., Tang, H. & Gilmour, D. S. Identification in vivo of different rate-limiting steps associated with transcriptional activators in the presence and absence of a GAGA element. Mol. Cell. Biol. 25, 3543–3552 (2005).
Lee, H., Kraus, K. W., Wolfner, M. F. & Lis, J. T. DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev. 6, 284–295 (1992).
Wu, C. H. et al. Molecular characterization of Drosophila NELF. Nucleic Acids Res. 33, 1269–1279 (2005).
Wu, C. H. et al. NELF and DSIF cause promoter proximal pausing on the hsp70 promoter in Drosophila. Genes Dev. 17, 1402–1414 (2003).
Park, J. M., Werner, J., Kim, J. M., Lis, J. T. & Kim, Y. J. Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol. Cell 8, 9–19 (2001).
Kim, J., Hake, S. B. & Roeder, R. G. The human homolog of yeast BRE1 functions as a transcriptional coactivator through direct activator interactions. Mol. Cell 20, 759–770 (2005).
Shilatifard, A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu. Rev. Biochem. 75, 243–269 (2006).
Lee, J. S. et al. Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell 131, 1084–1096 (2007).
Acknowledgements
We thank J.Conaway, C. Sato and S. Sato for helpful discussions regarding Mediator composition, and K. Lee and S. Venkatesh for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure/Table 1
Complexes involved in inducible gene expression are conserved from yeast to humans. (PDF 844 kb)
Related links
Glossary
- Chromatin
-
A nucleoprotein structure formed of repeating nucleosomal units in which 147 base pairs of DNA are wrapped around an octamer of histone proteins consisting of an H3–H4 tetramer flanked by two H2A–H2B dimers
- Co-activator
-
A protein that is recruited to promoters through interactions with transcriptional activators, and facilitates transcriptional activation through the recruitment of RNA polymerase II and the general transcription factors. Many co-activators also catalyse chromatin modifications that assist the kinetics of recruitment of the general transcription machinery.
- General transcription machinery
-
RNA polymerase II together with the general transcription factors TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH.
- Transcriptional activator
-
A sequence-specific DNA-binding protein that increases the rate of transcription by recruiting RNA polymerase II, either directly in prokaryotes or through co-activators in eukaryotes.
- Pre-initiation complex
-
A 44 polypeptide complex, which consists of RNA polymerase II and the general transcription factors TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH. Its formation at TATA-containing core promoters is nucleated by binding of TATA-binding protein (a component of TFIID) to the TATA element in the promoter DNA sequence.
- Acidic activation domain
-
The type of transactivation domain found in transcriptional activators such as Gal4. This domain contains a stretch of acidic amino acids and is required for interactions with co-activators.
- ATP-dependent nucleosome-remodelling complex
-
A transcriptional regulatory complex that uses the energy obtained from ATP hydrolysis to move or displace histone octamers from DNA.
Rights and permissions
About this article
Cite this article
Weake, V., Workman, J. Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet 11, 426–437 (2010). https://doi.org/10.1038/nrg2781
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2781
This article is cited by
-
Multi-omic single-cell velocity models epigenome–transcriptome interactions and improves cell fate prediction
Nature Biotechnology (2023)
-
HSF1 phosphorylation establishes an active chromatin state via the TRRAP–TIP60 complex and promotes tumorigenesis
Nature Communications (2022)
-
JMJD3 intrinsically disordered region links the 3D-genome structure to TGFβ-dependent transcription activation
Nature Communications (2022)
-
Myosin VI regulates the spatial organisation of mammalian transcription initiation
Nature Communications (2022)
-
Slc7a11 downregulation is rapidly reversed after cessation of competitive social stress in zebra finches
Molecular Biology Reports (2021)