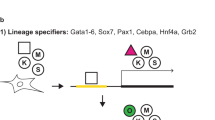
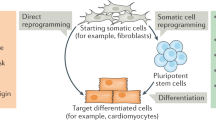
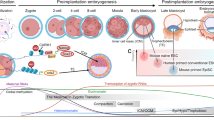
Key Points
-
This Review summarizes the progress to date on efforts to understand the molecular mechanisms underlying transcription factor-induced reprogramming of somatic cells to a pluripotent state (known as the induced pluripotent stem cell (iPSC) state).
-
We discuss potential causes for the inefficiency of the reprogramming process and highlight epigenetic barriers that cannot be easily overcome by the reprogramming factors.
-
New technologies and experiments are helping to determine the chronology of steps leading from loss of the somatic state to gain of pluripotency.
-
We are gaining mechanistic insights into the role of each of the reprogramming factors during the progression to pluripotency.
-
This Review also highlights the role of repressive chromatin as an inhibitor of reprogramming and discusses how chromatin states change at various stages of the process.
-
Studies of X chromosome inactivation and reactivation underscore the degree of chromatin remodelling that occurs during reprogramming. Failure to reactivate the somatically silent X chromosome in female human iPSCs is suggestive of differences in the developmental state between human and mouse embryonic stem cells (ESCs) and iPSCs.
-
Molecular comparisons of ESCs and iPSCs have uncovered various differences between these cell types, and some are informative about the mechanisms underlying the reprogramming process.
-
Finally, we speculate that a combination of novel technologies will accurately define all the molecular events of reprogramming.
Abstract
Induction of pluripotency by transcription factors has become a commonplace method to produce pluripotent stem cells. Great strides have been made in our understanding of the mechanism by which this occurs — particularly in terms of transcriptional and chromatin-based events — yet only a small part of the complete picture has been revealed. Understanding the mechanism of reprogramming to pluripotency will have important implications for improving the efficiency and quality of reprogramming and advancing therapeutic application of induced pluripotent stem cells. It will also help to reveal the machinery that stabilizes cell identity and to instruct the design of directed differentiation or lineage switching strategies. To inform the next phase in understanding reprogramming, we review the latest findings, highlight ongoing debates and outline future challenges.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). The first demonstration that expression of four pluripotency-related transcription factors can convert somatic cells to a pluripotent state (now known as the iPSC state).
Chin, M. H. et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5, 111–123 (2009).
Chin, M. H., Pellegrini, M., Plath, K. & Lowry, W. E. Molecular analyses of human induced pluripotent stem cells and embryonic stem cells. Cell Stem Cell 7, 263–269 (2010).
Hawkins, R. D. et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell 6, 479–491 (2010).
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007). The first characterization of XCI status in iPSC reprogramming. It demonstrated that mouse iPSCs are XaXa and can undergo random X-inactivation, indicating complete erasure of the memory of the prior inactive X chromosome.
Mikkelsen, T. S. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007).
Boland, M. J. et al. Adult mice generated from induced pluripotent stem cells. Nature 461, 91–94 (2009).
Kang, L., Wang, J., Zhang, Y., Kou, Z. & Gao, S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 5, 135–138 (2009).
Zhao, X. Y. et al. iPS cells produce viable mice through tetraploid complementation. Nature 461, 86–90 (2009).
Stadtfeld, M. et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 465, 175–181 (2010).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Park, I. H., Lerou, P. H., Zhao, R., Huo, H. & Daley, G. Q. Generation of human-induced pluripotent stem cells. Nature Protoc. 3, 1180–1186 (2008).
Lowry, W. E. et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl Acad. Sci. USA 105, 2883–2888 (2008).
Hanna, J. et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 (2009). This study addressed the inefficiency of reprogramming, argued for the first time that all cells of the starting population have the potential to be reprogrammed if given enough time in culture, and provided the first quantitative data on the actions of the reprogramming factors.
Smith, Z. D., Nachman, I., Regev, A. & Meissner, A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nature Biotech. 28, 521–526 (2010). The first high-resolution time-lapse imaging approach that enabled the retroactive tracking of faithful reprogramming events, and recognized that the path to the iPSC state begins with a transition to faster proliferation and a decrease in cell size immediately upon induction of the reprogramming factors.
Stadtfeld, M., Maherali, N., Breault, D. T. & Hochedlinger, K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230–240 (2008).
Brambrink, T. et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159 (2008). References 19 and 20 represent some of the first efforts to determine steps of reprogramming and define reprogramming factor dependence using an inducible system for reprogramming factor expression.
Li, R. et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7, 51–63 (2010).
Samavarchi-Tehrani, P . et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7, 64–77 (2010). References 21 and 22 identified the MET as an important step in the reprogramming of fibroblasts and showed that modulation of signalling pathways that affect the MET alter reprogramming efficiency.
Sridharan, R. et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell 136, 364–377 (2009). The first detailed description of the action of the reprogramming factors during induction of pluripotency, demonstrating differences between MYC and the other three reprogramming factors and defining barriers to the final step of reprogramming.
Silva, J. et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 6, e253 (2008).
Mali, P. et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 28, 713–720 (2010).
Shi, Y. et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3, 568–574 (2008).
Huangfu, D. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotech. 26, 795–797 (2008).
Huangfu, D. et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotech. 26, 1269–1275 (2008).
Liang, G., Taranova, O., Xia, K. & Zhang, Y. Butyrate promotes induced pluripotent stem cell generation. J. Biol. Chem. 285, 25516–25521 (2010).
Han, D. W. et al. Direct reprogramming of fibroblasts into epiblast stem cells. Nature Cell Biol. 13, 66–71 (2011).
Ichida, J. K. et al. A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing nanog. Cell Stem Cell 5, 491–503 (2009).
Maherali, N. & Hochedlinger, K. Tgfβ signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr. Biol. 19, 1718–1723 (2009).
Marson, A. et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3, 132–135 (2008).
Esteban, M. A. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 (2010).
Silva, J. et al. Nanog is the gateway to the pluripotent ground state. Cell 138, 722–737 (2009). A detailed analysis of the role of NANOG in establishing pluripotency, particularly in the reprogramming process. This paper demonstrated that NANOG is essential for generating iPSCs and is required during the final step of reprogramming, in which its overexpression also acts to enhance the process.
Ruiz, S. et al. A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cellidentity. Curr. Biol. 21, 45–52 (2010).
Polo, J. M. et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature Biotech. 28, 848–855 (2010).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010). References 37 and 38 provide functional evidence showing that an epigenetic memory of the target cell is present in mouse iPSCs, which can influence the differentiation behaviour of these cells and may be linked to DNA methylation.
Feng, B., Ng, J. H., Heng, J. C. & Ng, H. H. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell 4, 301–312 (2009).
Shao, L. et al. Generation of iPS cells using defined factors linked via the self-cleaving 2A sequences in a single open reading frame. Cell Res. 19, 296–306 (2009).
Gonzalez, F. et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc. Natl Acad. Sci. USA 106, 8918–8922 (2009).
Chang, C. W. et al. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells 27, 1042–1049 (2009).
Stadtfeld, M., Maherali, N., Borkent, M. & Hochedlinger, K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nature Methods 7, 53–55 (2010).
Woltjen, K. et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458, 766–770 (2009).
Carey, B. W. et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl Acad. Sci. USA 106, 157–162 (2009).
Wernig, M. et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nature Biotech. 26, 916–924 (2008).
Hockemeyer, D. et al. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell 3, 346–353 (2008).
Sommer, C. A. et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27, 543–549 (2009).
Maherali, N. et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 3, 340–345 (2008).
Carey, B. W., Markoulaki, S., Beard, C., Hanna, J. & Jaenisch, R. Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nature Methods 7, 56–59 (2009).
Stadtfeld, M., Brennand, K. & Hochedlinger, K. Reprogramming of pancreatic β cells into induced pluripotent stem cells. Curr. Biol. 18, 890–894 (2008).
Eminli, S. et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nature Genet. 41, 968–976 (2009).
Loh, Y. H. et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell 7, 15–19 (2010).
Seki, T. et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 7, 11–14 (2010).
Staerk, J. et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell 7, 20–24 (2010).
Hanna, J. et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133, 250–264 (2008).
Aoi, T. et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321, 699–702 (2008).
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G. & Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science 322, 945–949 (2008).
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T. & Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 322, 949–953 (2008).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
González, F., Boué, S. & Belmonte, J. C. I. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nature Rev. Genet. 12, 231–242 (2011).
Winkler, T. et al. No evidence for clonal selection due to lentiviral integration sites in human induced pluripotent stem cells. Stem Cells 28, 687–694 (2010).
Varas, F. et al. Fibroblast-derived induced pluripotent stem cells show no common retroviral vector insertions. Stem Cells 27, 300–306 (2009).
Chan, E. M. et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nature Biotech. 27, 1033–1037 (2009).
Koche, R. P. et al. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell 8, 96–105 (2011).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009).
Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136–1139 (2009).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 460, 1132–1135 (2009).
Marion, R. M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 (2009).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Krizhanovsky, V. & Lowe, S. W. Stem cells: the promises and perils of p53. Nature 460, 1085–1086 (2009).
Pereira, C. F. et al. ESCs require PRC2 to direct the successful reprogramming of differentiated cells toward pluripotency. Cell Stem Cell 6, 547–556 (2010).
Chou, Y. F. et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 135, 449–461 (2008).
Aasen, T. et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotech. 26, 1276–1284 (2008).
Markoulaki, S. et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nature Biotech. 27, 169–171 (2009).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotech. 26, 101–106 (2008).
Wernig, M., Meissner, A., Cassady, J. P. & Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2, 10–12 (2008).
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008).
Kim, J., Chu, J., Shen, X., Wang, J. & Orkin, S. H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132, 1049–1061 (2008).
Loh, Y. H. et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genet. 38, 431–440 (2006).
Jiang, J. et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nature Cell Biol. 10, 353–360 (2008).
Kim, J. et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 143, 313–324 (2010).
Rahl, P. B. et al. c-Myc regulates transcriptional pause release. Cell 141, 432–445 (2010).
Wang, J. et al. A protein interaction network for pluripotency of embryonic stem cells. Nature 444, 364–368 (2006).
Boyer, L. A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Theunissen, T. W. et al. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr. Biol. 21, 65–71 (2011).
Feldman, N. et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nature Cell Biol. 8, 188–194 (2006).
Li, W. et al. Generation of human induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells 27, 2992–3000 (2009).
Singhal, N. et al. Chromatin-remodeling components of the BAF complex facilitate reprogramming. Cell 141, 943–955 (2010).
Gaspar-Maia, A. et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature 460, 863–868 (2009).
Payer, B. & Lee, J. T. X chromosome dosage compensation: how mammals keep the balance. Annu. Rev. Genet. 42, 733–772 (2008).
Navarro, P. et al. Molecular coupling of Xist regulation and pluripotency. Science 321, 1693–1695 (2008).
Navarro, P. et al. Molecular coupling of Tsix regulation and pluripotency. Nature 468, 457–460 (2010).
Donohoe, M. E., Silva, S. S., Pinter, S. F., Xu, N. & Lee, J. T. The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature 460, 128–132 (2009).
Tchieu, J. et al. Female human iPS cells retain an inactive X-chromosome. Cell Stem Cell 7, 329–342 (2010).
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Guo, G. et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 (2009).
Bao, S. et al. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461, 1292–1295 (2009).
Hanna, J. et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl Acad. Sci. USA 107, 9222–9227 (2010). The first demonstration that human fibroblasts as well as human ESCs and iPSCs can be driven to a more primitive pluripotent state known as naive pluripotency. Based on X-inactivation status, morphology, gene expression and signalling dependence, this state is very similar to that of mouse ESCs.
Shen, Y. et al. X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc. Natl Acad. Sci. USA 105, 4709–4714 (2008).
Silva, S. S., Rowntree, R. K., Mekhoubad, S. & Lee, J. T. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc. Natl Acad. Sci. USA 105, 4820–4825 (2008).
Lengner, C. J. et al. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell 141, 872–883 (2010).
Ghosh, Z. et al. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS ONE 5, e8975 (2010).
Marchetto, M. C. et al. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS ONE 4, e7076 (2009).
Doi, A. et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature Genet. 41, 1350–1353 (2009).
Bock, C. et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Loewer, S. et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nature Genet. 42, 1113–1117 (2010). The authors uncovered large non-coding RNAs that are differentially expressed between human iPSCs and ESCs and demonstrated a role in reprogramming for one of these RNAs.
Lister, R. et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2 Feb 2011 (doi:10.1038/nature0 9798).
Guenther, M. G. et al. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell 7, 249–257 (2010).
Sommer, C. A. et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells 28, 64–74 (2010).
Roth, S. Y., Denu, J. M. & Allis, C. D. Histone acetyltransferases. Annu. Rev. Biochem. 70, 81–120 (2001).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384 (2009).
Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nature Rev. Drug Discov. 5, 769–784 (2006).
Chang, Y. et al. Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nature Struct. Mol. Biol. 16, 312–317 (2009).
Shi, Y. et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell 2, 525–528 (2008).
Wilson, K. D. et al. MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev. 18, 749–758 (2009).
Neveu, P. et al. MicroRNA profiling reveals two distinct p53-related human pluripotent stem cell states. Cell Stem Cell 7, 671–681 (2010).
Prigione, A., Fauler, B., Lurz, R., Lehrach, H. & Adjaye, J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28, 721–733 (2010).
Armstrong, L. et al. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28, 661–673 (2010).
Acknowledgements
K.P. is supported by the US National Institutes of Health (NIH) Director's Young Innovator Award (DP2OD001686) and a California Institute for Regenerative Medicine Young Investigator Award (RN1-00564). W.E.L. is the Maria Rowena Ross Professor of Cell Biology and Biochemistry and is supported by the NIH, The March of Dimes, and the Fuller Foundation. K.P. and W.E.L. are supported by the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California Los Angeles.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Induced pluripotent stem cells
-
Pluripotent cells that can be generated from many different types of somatic cells by expression of only a few pluripotency-related transcription factors, and that have properties of embryonic stem cells. They serve as an ideal platform to produce patient-specific pluripotent cells.
- Pluripotency
-
The ability of a cell to give rise to all cells of the embryo.
- Embryonic stem cells
-
Pluripotent cells derived from epiblast cells of the blastocyst upon explantation in culture.
- Reprogramming factors
-
Four transcription factors (OCT4, SOX2, KLF4 and MYC), first described by Shinya Yamanaka, that when forcibly expressed in somatic cells are capable of driving these cells into the induced pluripotent stem cell state.
- Epigenetic memory
-
The idea that at least a portion of somatic post-translational modifications on histones and DNA is retained despite reprogramming to a more immature state. This memory is thought to make cells adopt facets of physiology that are representative of a previous cellular state.
- Faithful reprogramming
-
Complete reprogramming to induced pluripotent stem cells, defined by endogenous expression of pluripotency-related genes (such as Nanog and Oct4). Presence of markers such as alkaline phosphatase or the surface antigen stage-specific embryonic antigen 1 (SSEA1), often used to assess reprogramming, mark partially as well as faithfully reprogrammed cells.
- Polycistronic cassette
-
DNA-containing sequence that codes for multiple genes expressed from a single promoter. These coding regions are sometimes separated by sequences that are cleaved during translation to produce individual protein products.
- Secondary reprogramming system
-
A system in which induced pluripotent stem cells are first generated from somatic cells with virally encoded inducible reprogramming factors, then differentiated again to obtain somatic cell populations that can express these factors in all cells and be used for secondary reprogramming experiments upon re-induction of the programming factors.
- Pre-iPSCs
-
Partially reprogrammed cells that arise in reprogramming cultures. They have efficiently silenced somatic genes but have not induced the endogenous pluripotency programme.
- NANOG
-
A transcription factor that is highly expressed in pluripotent cells and is essential for the establishment of embryonic stem cells but not for their maintenance. Although not belonging to the original Yamanaka set of reprogramming factors, NANOG overexpression enhances mouse cell reprogramming at the late step and has been used with OCT4 and SOX2 to reprogramme human cells.
- Mesenchymal-to-epithelial transition
-
Mesenchymal and epithelial cells are distinguished by, among other traits, their gene expression, morphology and cell adhesion properties. Transitions between these two states are thought to have key roles in development, cancer and, more recently, in reprogramming.
- Enhancers
-
DNA regions that positively control gene expression and that can be located upstream, downstream or even within the genes that they regulate. They are often bound by cell-type-specific transcription factors (such as OCT4 and NANOG in embryonic stem cells) and have a specific chromatin signature.
- X chromosome inactivation
-
Transcriptional silencing of one of the two X chromosomes in female mammalian cells, initiated during development when epiblast cells of the blastocyst differentiate.
- Naive pluripotent state
-
This stage of pluripotency is captured in vitro in the form of mouse embryonic stem cells or induced pluripotent stem cells. These cells can differentiate in vitro into many different cell types and, upon injection into blastocysts, can give rise to all tissues of the mouse, including the germ line.
- Primed pluripotent state
-
This stage of pluripotency is captured in vitro in the form of mouse epiblast stem cells and is considered developmentally more advanced than naive pluripotency, with respect to X-inactivation, signalling dependence, gene expression and the inability to contribute to chimeric animals. Human embryonic stem cells are more similar to mouse epiblast stem cells.
- Epiblast stem cells
-
Primed pluripotent cells derived from the post-implantation mouse epiblast of day 5.5–6.5 embryos.
Rights and permissions
About this article
Cite this article
Plath, K., Lowry, W. Progress in understanding reprogramming to the induced pluripotent state. Nat Rev Genet 12, 253–265 (2011). https://doi.org/10.1038/nrg2955
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2955
This article is cited by
-
Cellular senescence and acute kidney injury
Pediatric Nephrology (2022)
-
Dual-specificity Tyrosine Phosphorylation-regulated Kinase Inhibitor ID-8 Promotes Human Somatic Cell Reprogramming by Activating PDK4 Expression
Stem Cell Reviews and Reports (2022)
-
Transcriptome profiling of pluripotent pig embryonic stem cells originating from uni- and biparental embryos
BMC Research Notes (2020)
-
Acceleration of somatic cell reprogramming into the induced pluripotent stem cell using a mycosporine-like amino acid, Porphyra 334
Scientific Reports (2020)
-
Adapting machine-learning algorithms to design gene circuits
BMC Bioinformatics (2019)