Key Points
-
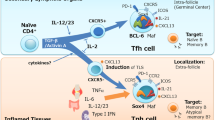
Germinal centres are crucial for long-lived antibody responses and are a fundamental feature of adaptive immune responses. The T cells that provide help to germinal-centre B cells were long considered to be T helper 2 (TH2) cells; however, non-polarized, CD4+ follicular B helper T (TFH) cells are now recognized to be the main providers of help to this B-cell subset.
-
TFH cells, as well as most B cells, express CXC-chemokine receptor 5 (CXCR5). This enables these cells to migrate towards the chemokine CXC-chemokine ligand 13 (CXCL13), which is expressed by follicular stromal cells, thereby facilitating T-cell–B-cell interactions.
-
In mice, differentiation into TFH cells has been shown to require signals from dendritic cells and CD4+CD3− accessory cells, including the ligation of CD28, CD30 and OX40. Antigen-specific B cells are also likely to influence TFH-cell commitment.
-
Molecules that are important for TFH-cell help to germinal-centre B cells include inducible T-cell co-stimulator (ICOS), CD40 ligand, interleukin-10 (IL-10) and signalling lymphocytic activation molecule (SLAM)-associated protein (SAP). The transcription factor B-cell lymphoma 6 (BCL-6) and the cytokine IL-21 are also likely to have important roles in TFH-cell differentiation and function.
-
The relationship of TFH cells to TH2 or TH1 cells is uncertain. TH1 and TH2 cytokines influence antibody responses and immunoglobulin class switching. It is possible that TH2 cells that receive appropriate signals enter the follicles, switch off IL-4 production and give rise to TFH cells.
-
Somatic hypermutation in germinal centres and the generation of new antibody specificities by B cells (which sometimes recognize self-antigens) requires that B cells receive selection signals from TFH cells that have themselves undergone stringent selection. Mutations in the roquin gene promote the differentiation or clonal expansion of self-reactive TFH cells, which leads to autoimmune disease. Therefore, control of T-cell help to germinal-centre B cells is a crucial checkpoint in peripheral T-cell tolerance.
-
Insufficient follicular T-cell help underlies certain immunodeficiencies, including X-linked lymphoproliferative syndrome (which is caused by a deficiency in SAP) and common variable immunodeficiency (which is caused by mutations in ICOS). In both of these conditions, there is impaired germinal-centre formation and an absence of memory B cells.
Abstract
T-cell help for B cells is essential for high-affinity antibody responses and B-cell memory. Recently, the identity of a discrete follicular population of T cells that has a crucial role in this process has become clearer. Similar to primed CD4+ T cells in the tonsils and memory CD4+ T cells in the peripheral blood, this follicular population of T cells expresses CXC-chemokine receptor 5 (CXCR5). Owing to their distinct homing preferences and helper function, these T cells differ from T helper 1 and T helper 2 cells and have been denoted follicular B helper T cells. Here, we outline the central role of this subset in normal and pathological immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416, 94–99 (2002).
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150 (2005).
MacLennan, I. C. et al. Extrafollicular antibody responses. Immunol. Rev. 194, 8–18 (2003).
MacLennan, I. C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Vinuesa, C. G. et al. Germinal centers without T cells. J. Exp. Med. 191, 485–494 (2000).
Velardi, A., Mingari, M. C., Moretta, L. & Grossi, C. E. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. J. Immunol. 137, 2808–2813 (1986). This was the first description of follicular T cells with a distinct cell-surface phenotype (CD57+) and a limited ability to produce IL-2.
Ansel, K. M., McHeyzer-Williams, L. J., Ngo, V. N., McHeyzer-Williams, M. G. & Cyster, J. G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999). This paper provided the first description of the in vivo upregulation of CXCR5 expression by antigen-specific T cells after immunization and before these T cells migrate into follicles.
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000). This paper, together with reference 10, was the first to describe and characterize T FH cells.
Kim, C. H. et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 193, 1373–1381 (2001). Similar to references 8 and 10, this study characterizes T FH cells, and it shows that helper function is contained in the CD57+ subset of T FH cells.
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Gunn, M. D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Cyster, J. G. et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176, 181–193 (2000).
Fuller, K. A., Kanagawa, O. & Nahm, M. H. T cells within germinal centers are specific for the immunizing antigen. J. Immunol. 151, 4505–4512 (1993).
Kearney, E. R., Pape, K. A., Loh, D. Y. & Jenkins, M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1, 327–339 (1994). This study shows that immunogenic and tolerogenic immunization regimes differ in their ability to induce the migration of T cells into follicles and indicates that this might be a crucial step for maintenance of peripheral T-cell tolerance.
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99 (1998). This paper provides visual documentation of cognate T-cell–B-cell interactions in vivo , showing how activated T cells and B cells move towards each other and become localized at the T-cell-zone–follicle boundary. Subsequent studies (including those reported in references 2 and 7) defined the chemokines and chemokine receptors that are responsible for this migration.
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-TH1/TH2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Kim, C. H. et al. Unique gene expression program of human germinal center T helper cells. Blood 104, 1952–1960 (2004). References 16 and 17 characterize the gene-expression profile of human T FH cells and identify numerous new molecules that are likely to be important for T FH -cell function. They show that T FH cells have a profile that is distinct from that of T H 1 or T H 2 cells. Interestingly, many of the genes that are identified concur with findings from a DNA-microarray-based study reported in reference 47.
Bowen, M. B., Butch, A. W., Parvin, C. A., Levine, A. & Nahm, M. H. Germinal center T cells are distinct helper-inducer T cells. Hum. Immunol. 31, 67–75 (1991). Before the discovery of CXCR5, this work provided the most complete description of the unique phenotype and cytokine-secretion pattern of follicular T cells.
Brachtel, E. F. et al. Differences in the germinal centres of palatine tonsils and lymph nodes. Scand. J. Immunol. 43, 239–247 (1996).
Mak, T. W. et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nature Immunol. 4, 765–772 (2003).
Rousset, F. et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl Acad. Sci. USA 89, 1890–1893 (1992).
Kim, J. R., Lim, H. W., Kang, S. G., Hillsamer, P. & Kim, C. H. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol. [online] 6, 3 (2005).
Liu, Y. J. et al. Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931 (1989).
Gaspal, F. M. et al. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J. Immunol. 174, 3891–3896 (2005).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Johansson-Lindbom, B., Ingvarsson, S. & Borrebaeck, C. A. Germinal centers regulate human TH2 development. J. Immunol. 171, 1657–1666 (2003).
Casamayor-Palleja, M., Khan, M. & MacLennan, I. C. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J. Exp. Med. 181, 1293–1301 (1995).
Fillatreau, S. & Gray, D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J. Exp. Med. 197, 195–206 (2003).
Lim, H. W., Hillsamer, P. & Kim, C. H. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-TH cells and GC-TH cell-driven B cell responses. J. Clin. Invest. 114, 1640–1649 (2004).
Cosgrove, D. et al. Mice lacking MHC class II molecules. Cell 66, 1051–1066 (1991).
Schaerli, P., Loetscher, P. & Moser, B. Induction of follicular homing precedes effector TH cell development. J. Immunol. 167, 6082–6086 (2001).
Campbell, D. J., Kim, C. H. & Butcher, E. C. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nature Immunol. 2, 876–881 (2001).
Walker, L. S. et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190, 1115–1122 (1999).
Kim, M. Y. et al. CD4+CD3− accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity 18, 643–654 (2003). This work describes a role for CD4+CD3− accessory cells (which are involved in lymph-node organization) in differentiation into T FH cells, by providing signals through OX40 and CD30.
Flynn, S., Toellner, K. M., Raykundalia, C., Goodall, M. & Lane, P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 188, 297–304 (1998).
Brocker, T. et al. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 29, 1610–1616 (1999).
Ebert, L. M., Horn, M. P., Lang, A. B. & Moser, B. B cells alter the phenotype and function of follicular-homing CXCR5+ T cells. Eur. J. Immunol. 34, 3562–3571 (2004).
Moser, B., Schaerli, P. & Loetscher, P. CXCR5+ T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 23, 250–254 (2002).
Stockinger, B., Zal, T., Zal, A. & Gray, D. B cells solicit their own help from T cells. J. Exp. Med. 183, 891–899 (1996).
Smith, K. M., Brewer, J. M., Rush, C. M., Riley, J. & Garside, P. In vivo generated TH1 cells can migrate to B cell follicles to support B cell responses. J. Immunol. 173, 1640–1646 (2004).
Secord, E. A. et al. Reconstitution of germinal center formation in nude mice with TH1 and TH2 clones. Cell. Immunol. 174, 173–179 (1996).
Randolph, D. A., Huang, G., Carruthers, C. J., Bromley, L. E. & Chaplin, D. D. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science 286, 2159–2162 (1999).
Dent, A. L., Hu-Li, J., Paul, W. E. & Staudt, L. M. T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc. Natl Acad. Sci. USA 95, 13823–13828 (1998).
Cunningham, A. F. et al. Pinpointing IL-4-independent acquisition and IL-4-influenced maintenance of TH2 activity by CD4 T cells. Eur. J. Immunol. 34, 686–694 (2004).
Dent, A. L., Doherty, T. M., Paul, W. E., Sher, A. & Staudt, L. M. BCL-6-deficient mice reveal an IL-4-independent, STAT6-dependent pathway that controls susceptibility to infection by Leishmania major. J. Immunol. 163, 2098–2103 (1999).
Ozaki, K. et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173, 5361–5371 (2004).
Vinuesa, C. G. et al. A novel RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005). This work identifies a novel gene, roquin, that negatively regulates T FH -cell differentiation, and it shows a link between T FH cells and autoantibody-mediated autoimmune disease.
Quezada, S. A., Jarvinen, L. Z., Lind, E. F. & Noelle, R. J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 (2004).
Casamayor-Palleja, M., Feuillard, J., Ball, J., Drew, M. & MacLennan, I. C. Centrocytes rapidly adopt a memory B cell phenotype on co-culture with autologous germinal centre T cell-enriched preparations. Int. Immunol. 8, 737–744 (1996).
Han, S. et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 155, 556–567 (1995).
Gulino, A. V. & Notarangelo, L. D. Hyper IgM syndromes. Curr. Opin. Rheumatol. 15, 422–429 (2003).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999). This work identifies the co-stimulatory molecule ICOS and shows that the highest levels of ICOS are expressed by germinal-centre T cells.
Lohning, M. et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J. Exp. Med. 197, 181–193 (2003). This paper describes a positive correlation between the level of expression of ICOS by mouse CD4+ T cells and the capacity of these cells to produce IL-10.
Rennick, D. M., Fort, M. M. & Davidson, N. J. Studies with IL-10−/− mice: an overview. J. Leukoc. Biol. 61, 389–396 (1997).
Liang, L., Porter, E. M. & Sha, W. C. Constitutive expression of the B7h ligand for inducible costimulator on naive B cells is extinguished after activation by distinct B cell receptor and interleukin 4 receptor-mediated pathways and can be rescued by CD40 signaling. J. Exp. Med. 196, 97–108 (2002).
Aicher, A. et al. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 164, 4689–4696 (2000).
Dong, C., Temann, U. A. & Flavell, R. A. Critical role of inducible costimulator in germinal center reactions. J. Immunol. 166, 3659–3662 (2001).
McAdam, A. J. et al. ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 (2001).
Tafuri, A. et al. ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 (2001). References 58 and 59 show that ICOS has an essential role in T-cell help for B cells and in immunoglobulin class switching.
Grimbacher, B. et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nature Immunol. 4, 261–268 (2003). This paper reports on a deficiency in ICOS in humans and the resultant phenotype.
Lane, P. et al. B cell function in mice transgenic for mCTLA4-Hγ1: lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J. Exp. Med. 179, 819–830 (1994).
Ferguson, S. E., Han, S., Kelsoe, G. & Thompson, C. B. CD28 is required for germinal center formation. J. Immunol. 156, 4576–4581 (1996).
Parrish-Novak, J. et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408, 57–63 (2000).
Pene, J. et al. IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172, 5154–5157 (2004).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002). References 64 and 65 report the potent effects of IL-21 on the differentiation of mouse and human B cells into antibody-secreting cells. In addition, reference 65 shows that IL-21 has a considerable influence on the regulation of B-cell function in vivo and that it cooperates with IL-4.
Parrish-Novak, J., Foster, D. C., Holly, R. D. & Clegg, C. H. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J. Leukoc. Biol. 72, 856–863 (2002).
Ho, I. C. & Glimcher, L. H. Transcription: tantalizing times for T cells. Cell 109, S109–S120 (2002).
Allman, D. et al. BCL-6 expression during B-cell activation. Blood 87, 5257–5268 (1996).
Cattoretti, G. et al. BCL-6 protein is expressed in germinal-center B cells. Blood 86, 45–53 (1995).
Onizuka, T. et al. BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood 86, 28–37 (1995).
Ree, H. J. et al. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum. Pathol. 30, 403–411 (1999).
Shaffer, A. L. et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13, 199–212 (2000).
Ye, B. H. et al. The BCL-6 proto-oncogene controls germinal-centre formation and TH2-type inflammation. Nature Genet. 16, 161–170 (1997).
Kusam, S., Toney, L. M., Sato, H. & Dent, A. L. Inhibition of TH2 differentiation and GATA-3 expression by BCL-6. J. Immunol. 170, 2435–2441 (2003).
Nichols, K. E., Ma, C. S., Cannons, J., Schwartzberg, P. L. & Tangye, S. G. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol. Rev. 203, 180–199 (2005).
Sayos, J. et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395, 462–469 (1998).
Nichols, K. E. et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl Acad. Sci. USA 95, 13765–13770 (1998).
Coffey, A. J. et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nature Genet. 20, 129–135 (1998). References 76–78 identify the genetic defect that underlies the human immunodeficiency disease XLP.
Latour, S. et al. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nature Cell Biol. 5, 149–154 (2003).
Tangye, S. G., Phillips, J. H. & Lanier, L. L. The CD2-subset of the Ig superfamily of cell surface molecules: receptor–ligand pairs expressed by NK cells and other immune cells. Semin. Immunol. 12, 149–157 (2000).
Sidorenko, S. P. & Clark, E. A. The dual-function CD150 receptor subfamily: the viral attraction. Nature Immunol. 4, 19–24 (2003).
Latour, S. & Veillette, A. Molecular and immunological basis of X-linked lymphoproliferative disease. Immunol. Rev. 192, 212–224 (2003).
Engel, P., Eck, M. J. & Terhorst, C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nature Rev. Immunol. 3, 813–821 (2003).
Nichols, K. E. et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nature Med. 11, 340–345 (2005).
Martin, M. et al. CD84 functions as a homophilic adhesion molecule and enhances IFN-γ secretion: adhesion is mediated by Ig-like domain 1. J. Immunol. 167, 3668–3676 (2001).
Tangye, S. G., van de Weerdt, B. C., Avery, D. T. & Hodgkin, P. D. CD84 is up-regulated on a major population of human memory B cells and recruits the SH2 domain containing proteins SAP and EAT-2. Eur. J. Immunol. 32, 1640–1649 (2002).
Romero, X. et al. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4). Tissue Antigens 64, 132–144 (2004).
Romero, X. et al. CD229 (Ly9) lymphocyte cell surface receptor interacts homophilically through its N-terminal domain and relocalizes to the immunological synapse. J. Immunol. 174, 7033–7042 (2005).
Crotty, S., Kersh, E. N., Cannons, J., Schwartzberg, P. L. & Ahmed, R. SAP is required for generating long-term humoral immunity. Nature 421, 282–287 (2003). This paper shows that SAP is required for the formation of germinal centres in response to viral infection, and it also shows the subsequent generation of long-lived effector memory cells and plasma cells (also see reference 121).
Ma, C. S. et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J. Clin. Invest. 115, 1049–1059 (2005). This study shows that CD4+ T cells from patients with XLP are deficient in their ability to produce IL-10, to provide help to B cells and to upregulate ICOS expression. It also shows that these defects can be overcome by restoring expression of SAP.
Wang, N. et al. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med. 199, 1255–1264 (2004).
Howie, D. et al. The SLAM family receptor Ly108 controls T cell and neutrophil functions. J. Immunol. 174, 5931–5935 (2005).
Cannons, J. L. et al. SAP regulates TH2 differentiation and PKC-θ-mediated activation of NF-κB1. Immunity 21, 693–706 (2004).
Wu, C. et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nature Immunol. 2, 410–414 (2001).
Czar, M. J. et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc. Natl Acad. Sci. USA 98, 7449–7454 (2001). References 94 and 95 report the phenotype of SAP-deficient mice, showing that they have an impairment in IL-4 production by CD4+ T cells.
Wong, S. C., Oh, E., Ng, C. H. & Lam, K. P. Impaired germinal center formation and recall T-cell-dependent immune responses in mice lacking the costimulatory ligand B7-H2. Blood 102, 1381–1388 (2003).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005).
Samuels, J., Ng, Y. S., Coupillaud, C., Paget, D. & Meffre, E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 201, 1659–1667 (2005).
Adelstein, S. et al. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science 251, 1223–1225 (1991).
Goodnow, C., Sprent, J., Fazekas de St Groth, B. & Vinuesa, C. G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435, 590–597 (2005).
Winkler, T. H., Fehr, H. & Kalden, J. R. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur. J. Immunol. 22, 1719–1728 (1992).
Luzina, I. G. et al. Spontaneous formation of germinal centers in autoimmune mice. J. Leukoc. Biol. 70, 578–584 (2001).
Armengol, M. P. et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am. J. Pathol. 159, 861–873 (2001).
Stott, D. I., Hiepe, F., Hummel, M., Steinhauser, G. & Berek, C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J. Clin. Invest. 102, 938–946 (1998).
Salomonsson, S. et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum. 48, 3187–3201 (2003).
Pugh-Bernard, A. E. et al. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest. 108, 1061–1070 (2001).
Smith, K. M., McAskill, F. & Garside, P. Orally tolerized T cells are only able to enter B cell follicles following challenge with antigen in adjuvant, but they remain unable to provide B cell help. J. Immunol. 168, 4318–4325 (2002).
Smith, K. M. et al. Inducible costimulatory molecule–B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, TH1, and TH2 T cells. J. Immunol. 170, 2310–2315 (2003).
Huang, W. et al. The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum. 46, 1554–1562 (2002).
Boumpas, D. T. et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 48, 719–727 (2003).
Grammer, A. C. et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154–CD40 interactions. J. Clin. Invest. 112, 1506–1520 (2003). This paper reports the successful treatment of several patients with SLE, using a blocking monoclonal antibody specific for CD40L.
Hutloff, A. et al. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 50, 3211–3220 (2004).
Okamoto, T. et al. Expression and function of the co-stimulator H4/ICOS on activated T cells of patients with rheumatoid arthritis. J. Rheumatol. 30, 1157–1163 (2003).
Dong, C. & Nurieva, R. I. Regulation of immune and autoimmune responses by ICOS. J. Autoimmun. 21, 255–260 (2003).
Nurieva, R. I., Treuting, P., Duong, J., Flavell, R. A. & Dong, C. Inducible costimulator is essential for collagen-induced arthritis. J. Clin. Invest. 111, 701–706 (2003).
Iwai, H. et al. Amelioration of collagen-induced arthritis by blockade of inducible costimulator–B7 homologous protein costimulation. J. Immunol. 169, 4332–4339 (2002).
Scott, B. G. et al. ICOS is essential for the development of experimental autoimmune myasthenia gravis. J. Neuroimmunol. 153, 16–25 (2004).
Iwai, H. et al. Involvement of inducible costimulator–B7 homologous protein costimulatory pathway in murine lupus nephritis. J. Immunol. 171, 2848–2854 (2003).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004). This paper provides evidence that IL-21 contributes to autoimmune-disease development in mice, particularly in circumstances that involve lymphopaenia.
Hron, J. D., Caplan, L., Gerth, A. J., Schwartzberg, P. L. & Peng, S. L. SH2D1A regulates T-dependent humoral autoimmunity. J. Exp. Med. 200, 261–266 (2004). A deficiency in SAP protects mice from SLE-like disease and impairs T-cell-dependent antibody responses, owing to defective germinal-centre formation.
Seo, S. J. et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity 16, 535–546 (2002).
Walker, L. S. et al. Established T cell-driven germinal center B cell proliferation is independent of CD28 signaling but is tightly regulated through CTLA-4. J. Immunol. 170, 91–98 (2003).
Legler, D. F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Mazzucchelli, L. et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Invest. 104, R49–R54 (1999).
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Carlsen, H. S., Baekkevold, E. S., Morton, H. C., Haraldsen, G. & Brandtzaeg, P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood 104, 3021–3027 (2004).
Perrier, P. et al. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J. Immunol. 172, 7031–7042 (2004).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Amft, N. et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren's syndrome. Arthritis Rheum. 44, 2633–2641 (2001).
Takemura, S. et al. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167, 1072–1080 (2001).
Carlsen, H. S., Baekkevold, E. S., Johansen, F. E., Haraldsen, G. & Brandtzaeg, P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut 51, 364–371 (2002).
Mori, M. et al. BCA-1, a B-cell chemoattractant signal, is constantly expressed in cutaneous lymphoproliferative B-cell disorders. Eur. J. Cancer 39, 1625–1631 (2003).
Chan, C. C., Shen, D., Hackett, J. J., Buggage, R. R. & Tuaillon, N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology 110, 421–426 (2003).
Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004).
Corcione, A. et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl Acad. Sci. USA 101, 11064–11069 (2004).
Forster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Forster, R., Emrich, T., Kremmer, E. & Lipp, M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84, 830–840 (1994).
Hargreaves, D. C. et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194, 45–56 (2001). This paper reports on the altered responsiveness of B cells as they differentiate into plasma cells, and it highlights the crucial role of CXCL12 and its receptor CXCR4 in the positioning and homing of plasma cells to the splenic red pulp and the bone marrow.
Ellyard, J. E., Avery, D. T., Mackay, C. R. & Tangye, S. G. Contribution of stromal cells to the migration, function and retention of plasma cells in human spleen: potential roles of CXCL12, IL-6 and CD54. Eur. J. Immunol. 35, 699–708 (2005).
Acknowledgements
This work was supported by the National Health and Medical Research Council (Australia), the Wellcome Trust (United Kingdom) and the Cooperative Research Centre for Asthma (Australia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- RED PULP
-
The blood-filtering part of the spleen that is formed by sinuses. The sinuses are usually filled with blood and consist of reticular fibres, which are lined with specialized macrophages that form part of the reticuloendothelial system. The main function of the red pulp is to destroy senescent erythrocytes. Long-lived plasma cells also localize to this region of the spleen.
- GERMINAL CENTRE
-
A lymphoid structure that arises within follicles after immunization with, or exposure to, a T-cell-dependent antigen. It is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells.
- SOMATIC HYPERMUTATION
-
Point mutations that occur in cycling centroblasts and are targeted to the immunoglobulin variable-region gene segments. Some mutations might generate a binding site with increased affinity for the specific antigen, but others can lead to loss of antigen recognition by the B-cell receptor and generation of a self-reactive B-cell receptor.
- CLASS-SWITCH RECOMBINATION
-
The process by which proliferating B cells rearrange their DNA to switch from expressing IgM (or another class of immunoglobulin) to expressing a different immunoglobulin heavy-chain constant region, thereby producing antibody with different effector functions.
- CENTROBLAST
-
A proliferating germinal-centre B cell, which undergoes somatic hypermutation and immunoglobulin class switching.
- CENTROCYTE
-
The non-dividing progeny of a centroblast. These cells need to be selected on the basis of affinity for antigen, following interaction with immune complexes that are associated with follicular dendritic cells, and ability to elicit help from follicular B helper T (TFH) cells.
- MANTLE ZONE
-
The area of a secondary follicle that surrounds the germinal centre and contains IgD+ naive, resting B cells.
- LIGHT ZONE
-
The area of the germinal centre that is most distant from the T-cell zone. These areas contain a rich network of follicular dendritic cells, which hold antigen on their surface. They are filled with centrocytes and small numbers of follicular T cells.
- CHEMOATTRACTANT-RECEPTOR HOMOLOGOUS MOLECULE EXPRESSED BY TH2 CELLS
-
(CRTH2). A cell-surface marker for human T helper 2 (TH2) cells.
- REGULATORY T CELLS
-
(TReg cells). A small population of CD4+ T cells that expresses the transcription factor forkhead box P3 (FOXP3) and has regulatory (that is, suppressor) activity towards other T cells that are stimulated through their T-cell receptor. An absence of TReg cells or their dysfunction is associated with severe autoimmunity.
- CENTRAL MEMORY T CELLS
-
Memory T cells that express L-selectin and CC-chemokine receptor 7 (CCR7) and have the capacity to circulate from the blood to the secondary lymphoid organs. They have a non-polarized differentiation state in that they secrete interleukin-2 but not interferon-γ or interleukin-4; however, on restimulation, they rapidly differentiate into cytokine-producing effector cells.
- SRC HOMOLOGY 2 DOMAIN
-
(SH2 domain). A protein domain of ∼100 amino-acid residues that is found in many intracellular signal-transducing proteins. It interacts with high affinity with phosphotyrosine-containing target peptide sequences in a sequence-specific, and usually phosphorylation-dependent, manner.
- SYSTEMIC LUPUS ERYTHEMATOSUS
-
(SLE). An autoimmune disorder in which antibodies are raised against one's own DNA and form immune complexes that cause end-organ damage. It classically presents with a butterfly-shaped rash across the cheek bones, which causes a wolf-like appearance (lupus being Latin for wolf).
- COMPLETE FREUND'S ADJUVANT
-
(CFA). An oil that contains an emulsifying agent and killed mycobacteria, which increase the immune response to an immunogen. For administration, a water-in-oil emulsion is made with a solution that contains the immunogen of interest.
- NON-OBESE DIABETIC MICE
-
(NOD mice). A mouse strain that has a polygenic susceptibility to spontaneous development of autoimmune, type 1 diabetes. The main component of susceptibility is the unique MHC haplotype H2g7.
- MOLECULAR MIMICRY
-
A mechanism by which a peptide from a foreign antigen that is presented to a T cell closely resembles part of a self-protein, thereby triggering an autoimmune reaction.
- SANROQUE MICE
-
An autoimmune strain of mice that carries a loss-of-function mutation in the gene roquin. These mice have a T-cell-mediated systemic-lupus-erythematosus-like syndrome and severe autoimmune diabetes when on a susceptible genetic background.
- EFFECTOR MEMORY T CELLS
-
Cells that have an L-selectin− CCR7 phenotype. They have immediate effector functions, including rapid production of cytokines (such as interferon-γ or interleukin-4), and they migrate to sites of inflammation, such as the skin and the gut.
Rights and permissions
About this article
Cite this article
Vinuesa, C., Tangye, S., Moser, B. et al. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol 5, 853–865 (2005). https://doi.org/10.1038/nri1714
Issue Date:
DOI: https://doi.org/10.1038/nri1714
This article is cited by
-
Spatiotemporal resolution of germinal center Tfh cell differentiation and divergence from central memory CD4+ T cell fate
Nature Communications (2023)
-
RBP–RNA interactions in the control of autoimmunity and autoinflammation
Cell Research (2023)
-
The HDAC inhibitor zabadinostat is a systemic regulator of adaptive immunity
Communications Biology (2023)
-
Pathological count of IgG4-positive plasmacytes suggests extraophthalmic involvement and relapse in patients with IgG4-related ophthalmic disease: a retrospective study
Arthritis Research & Therapy (2022)
-
A follicular regulatory Innate Lymphoid Cell population impairs interactions between germinal center Tfh and B cells
Communications Biology (2021)