Abstract
Chronic immunological processes that underlie persistent viral infections and autoimmune disorders such as multiple sclerosis can be relapsing–remitting in nature. The progressive loss of β-cell mass during the development of autoimmune type 1 diabetes (T1D) can also be non-linear, but the exact nature and kinetics of the immunological processes that govern T1D are not known. Here, we propose that the immunological process that is at the root of T1D is relapsing–remitting in nature and discuss the unresolved controversies and therapeutic implications of this hypothesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann. Intern. Med. 128, 517–523 (1998).
Lundberg, K. et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis. Res. Ther. 7, R458–R467 (2005).
Makrygiannakis, D. et al. Citrullination is an inflammation-dependent process. Ann. Rheum. Dis. 65, 1219–1222 (2006).
Yu, M., Johnson, J. M. & Tuohy, V. K. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J. Exp. Med. 183, 1777–1788 (1996).
Lehmann, P. V., Sercarz, E. E., Forsthuber, T., Dayan, C. M. & Gammon, G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol. Today 14, 203–208 (1993).
Campbell, I. L., Kay, T. W., Oxbrow, L. & Harrison, L. C. Essential role for interferon-γ and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J. Clin. Invest. 87, 739–742 (1991).
Doyle, H. A. & Mamula, M. J. Posttranslational modifications of self-antigens. Ann. NY Acad. Sci. 1050, 1–9 (2005).
Eizirik, D. L. Interleukin-1 induced impairment in pancreatic islet oxidative metabolism of glucose is potentiated by tumor necrosis factor. Acta. Endocrinol. (Copenh) 119, 321–325 (1988).
Eizirik, D. L. & Darville, M. I. β-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes 50 (Suppl. 1), 64–69 (2001).
Eizirik, D. L., Welsh, M., Strandell, E., Welsh, N. & Sandler, S. Interleukin-1β depletes insulin messenger ribonucleic acid and increases the heat shock protein hsp70 in mouse pancreatic islets without impairing the glucose metabolism. Endocrinology 127, 2290–2297 (1990).
Sercarz, E. E. Driver clones and determinant spreading. J. Autoimmun. 14, 275–277 (2000).
Lacher, M. D. et al. Transforming growth factor-β receptor inhibition enhances adenoviral infectability of carcinoma cells via up-regulation of coxsackie and adenovirus receptor in conjunction with reversal of epithelial-mesenchymal transition. Cancer Res. 66, 1648–1657 (2006).
Dotta, F. et al. Coxsackie B4 virus infection of β cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl Acad. Sci. USA 104, 5115–5120 (2007).
Kaniuk, N. A. et al. Ubiquitinated-protein aggregates form in pancreatic β-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes 56, 930–939 (2007).
Pugliese, A. Central and peripheral autoantigen presentation in immune tolerance. Immunology 111, 138–146 (2004).
Bonifacio, E., Scirpoli, M., Kredel, K., Fuchtenbusch, M. & Ziegler, A. G. Early autoantibody responses in prediabetes are IgG1 dominated and suggest antigen-specific regulation. J. Immunol. 163, 525–532 (1999).
Kent, S. C. et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature 435, 224–228 (2005).
Nakayama, M. et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005).
Krishnamurthy, B. et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 116, 3258–3265 (2006).
Yu, L. et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J. Clin. Endocrinol. Metab. 81, 4264–4267 (1996).
Skyler, J. S. et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care 28, 1068–1076 (2005).
Lindley, S. et al. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes 54, 92–99 (2005).
Arif, S. et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 113, 451–463 (2004).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12, 431–440 (2000).
Tang, Q. et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nature Immunol. 7, 83–92 (2006).
Tang, Q. et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465 (2004).
Green, E. A., Gorelik, L., McGregor, C. M., Tran, E. H. & Flavell, R. A. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-β–TGF-β receptor interactions in type 1 diabetes. Proc. Natl Acad. Sci. USA 100, 10878–10883 (2003).
Chatenoud, L., Thervet, E., Primo, J. & Bach, J. F. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 91, 123–127 (1994).
Peng, Y., Laouar, Y., Li, M. O., Green, E. A. & Flavell, R. A. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl Acad. Sci. USA 101, 4572–4577 (2004).
Belghith, M. et al. TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nature Med. 9, 1202–1208 (2003).
Sreenan, S. et al. Increased β-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 48, 989–996 (1999).
Sherry, N. A. et al. Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 55, 3238–3245 (2006).
Zhao, R. Y. & Elder, R. T. Viral infections and cell cycle G2/M regulation. Cell Res. 15, 143–149 (2005).
Chase, H. P. et al. Redefining the clinical remission period in children with type 1 diabetes. Pediatr. Diabetes 5, 16–19 (2004).
Muhammad, B. J., Swift, P. G., Raymond, N. T. & Botha, J. L. Partial remission phase of diabetes in children younger than age 10 years. Arch. Dis. Child. 80, 367–369 (1999).
Bober, E., Dundar, B. & Buyukgebiz, A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. J. Pediatr. Endocrinol. Metab. 14, 435–441 (2001).
Robles, D. T. et al. Millennium award recipient contribution. Identification of children with early onset and high incidence of anti-islet autoantibodies. Clin. Immunol. 102, 217–224 (2002).
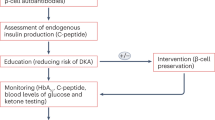
Palmer, J. P. et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 53, 250–264 (2004).
Schober, E., Schernthaner, G., Frisch, H. & Fink, M. β-cell function recovery is not the only factor responsible for remission in type I diabetics: evaluation of C-peptide secretion in diabetic children after first metabolic recompensation and at partial remission phase. J. Endocrinol. Invest. 7, 507–512 (1984).
Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346, 1685–1691 (2002).
Imagawa, A. et al. Immunological abnormalities in islets at diagnosis paralleled further deterioration of glycaemic control in patients with recent-onset type I (insulin-dependent) diabetes mellitus. Diabetologia 42, 574–578 (1999).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Raz, I. et al. β-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 358, 1749–1753 (2001).
Herold, K. C. et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54, 1763–1769 (2005).
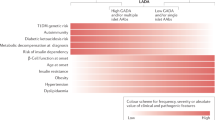
Agardh, C. D. et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J. Diabetes Complicat. 19, 238–246 (2005).
Seyfert-Margolis, V. et al. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes 55, 2588–2594 (2006).
Bresson, D. et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 116, 1371–1381 (2006).
Sherry, N. et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 mAb by enhancing recovery of β cells. Endocrinology 148, 5136–5144 (2007).
Ogawa, N., List, J. F., Habener, J. F. & Maki, T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes 53, 1700–1705 (2004).
Fife, B. T. et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1–PD-L1 pathway. J. Exp. Med. 203, 2737–2747 (2006).
Achenbach, P., Bonifacio, E., Koczwara, K. & Ziegler, A. G. Natural history of type 1 diabetes. Diabetes 54 (Suppl. 2), 25–31 (2005).
McMahon, E. J., Bailey, S. L., Castenada, C. V., Waldner, H. & Miller, S. D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nature Med. 11, 335–339 (2005).
Author information
Authors and Affiliations
Corresponding authors
Related links
Glossary
- Connecting peptide
-
(C-peptide). Insulin is synthesized by β-cells as a hormone precursor known as pro-insulin. When released from the pancreas into the blood, pro-insulin is cleaved into insulin and a small peptide known as C-peptide. C-peptide can be used as a measure of endogenous insulin secretion (one C-peptide is released for each insulin molecule secreted).
- Cryptic epitope
-
A cryptic epitope is an antigenic peptide generated or 'unmasked' under altered conditions, such as inflammation or autophagy. When cryptic epitopes become visible to the immune system they become good candidates for eliciting an immune response responsible for autoimmune disease.
- Epitope spreading
-
The de novo activation of (autoreactive) T cells by antigens that have been released after damage of target cells (in this context, β-cells) has occurred.
- Glycaemic control
-
This is a medical term that refers to the typical levels of blood sugar (glucose) in a person with diabetes mellitus. Good glycaemic control, in the sense of a 'target' for treatment, has become an important goal of diabetes care.
- Honeymoon phase
-
This is a partial remission phase in type 1 diabetes that usually begins within weeks of diagnosis, initiation of subcutaneous insulin therapy and correction of hyperglycaemia. It is characterized by a temporary reduction in insulin requirements (patients need less than 0.5 units per kg per day of insulin) and improved glycaemic control.
- Intramolecular and intermolecular spreading
-
A term that refers to the recognition of new determinants or epitopes by T or B cells during the development of an (auto)immune response.
Rights and permissions
About this article
Cite this article
von Herrath, M., Sanda, S. & Herold, K. Type 1 diabetes as a relapsing–remitting disease?. Nat Rev Immunol 7, 988–994 (2007). https://doi.org/10.1038/nri2192
Issue Date:
DOI: https://doi.org/10.1038/nri2192
This article is cited by
-
Precision Medicine in Type 1 Diabetes
Journal of the Indian Institute of Science (2023)
-
RNA aptamers specific for transmembrane p24 trafficking protein 6 and Clusterin for the targeted delivery of imaging reagents and RNA therapeutics to human β cells
Nature Communications (2022)
-
Immunoregulated insulitis and slow-progressing type 1 diabetes after duodenopancreatectomy
Diabetologia (2021)
-
Transplantation of MHC-mismatched mouse embryonic stem cell-derived thymic epithelial progenitors and MHC-matched bone marrow prevents autoimmune diabetes
Stem Cell Research & Therapy (2019)
-
Toll-like receptor 4 inhibition prevents autoimmune diabetes in NOD mice
Scientific Reports (2019)