Key Points
-
The activation of T cells is controlled by T cell receptor (TCR) engagement with peptide antigen presented on MHC molecules (peptide–MHC complexes). The process is uniquely challenging because of the very low abundance of foreign agonist peptide compared with the very high abundance of self peptide, leading to difficulties with discrimination of signal from noise.
-
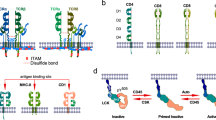
TCR engagement leads to alterations in the cytoplasmic portions of the associated CD3 subunits, including phosphorylation of tyrosine residues, a process termed TCR triggering. The mechanisms that have been proposed for TCR triggering include aggregation, conformational change and segregation of the TCR.
-
The pseudodimer model postulates that self peptide–MHC complexes cooperate with foreign peptide–MHC complexes to induce TCR aggregation. Recent evidence that a large proportion of self peptide–MHC complexes can bind the TCR provides strong support for this model.
-
There has been renewed interest in conformational change models following the demonstration of conformational changes in the CD3 cytoplasmic domains and the finding that these changes could be induced by the pulling mechanical force that the TCR is subjected to upon peptide–MHC binding.
-
There is also evidence that segregation induced by ectodomain size differences or partitioning into membrane microdomains contributes to TCR triggering.
-
A model is proposed that incorporates and reconciles these aggregation, conformational change and segregation models, and postulates a role for TCR microclusters in signal/noise discrimination.
Abstract
There is considerable controversy about the mechanism of T cell receptor (TCR) triggering, the process by which the TCR tranduces signals across the plasma membrane after binding to its ligand (an agonist peptide complexed with an MHC molecule). Three main types of mechanism have been proposed, which involve aggregation, conformational change and segregation. Here, we review recently published evidence for each type of mechanism and conclude that all three may be involved. This complexity may reflect the uniquely demanding nature of TCR-mediated antigen recognition, which requires the detection of a very weak 'signal' (very rare foreign peptide–MHC ligands) in the presence of considerable 'noise' (abundant self peptide–MHC molecules).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Weiss, A. & Littman, D. R. Signal transduction by lymphocyte antigen receptors. Cell 76, 263–274 (1994).
Weiss, A. & Samelson, L. E. in Fundamental Immunology (ed. Paul, W. E.) 321–364 (Lippincott Williams & Wilkins, Philadelphia, 2003).
Gil, D., Schamel, W. W., Montoya, M., Sanchez-Madrid, F. & Alarcon, B. Recruitment of Nck by CD3ɛ reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109, 901–912 (2002).
Gil, D., Schrum, A. G., Alarcon, B. & Palmer, E. T cell receptor engagement by peptide–MHC ligands induces a conformational change in the CD3 complex of thymocytes. J. Exp. Med. 201, 517–522 (2005).
Risueno, R. M., Schamel, W. W. & Alarcon, B. T cell receptor engagement triggers its CD3ɛ and CD3ζ subunits to adopt a compact, locked conformation. PLoS ONE 3, e1747 (2008).
Aivazian, D. & Stern, L. J. Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nature Struct. Biol. 7, 1023–1026 (2000).
Xu, C. et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3ɛ cytoplasmic tyrosine-based motif. Cell 135, 702–713 (2008). This paper provides evidence that the cytoplasmic domain of CD3ɛ associates with the plasma membrane of a cell and that the ITAM tyrosine residues are buried within the membrane, where presumably they are inaccessible to kinases.
Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4, 565–571 (1996).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Purbhoo, M. A., Irvine, D. J., Huppa, J. B. & Davis, M. M. T cell killing does not require the formation of a stable mature immunological synapse. Nature Immunol. 5, 524–530 (2004).
Ernst, B., Lee, D., Chang, J. M., Sprent, J. & Surh, C. D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Goldrath, A. W. & Bevan, M. J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190 (1999).
Viret, C., Wong, F. S. & Janeway, C. A. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10, 559–568 (1999).
Palmer, E. & Naeher, D. Affinity threshold for thymic selection through a T-cell receptor–co-receptor zipper. Nature Rev. Immunol. 9, 207–213 (2009).
Huse, M. et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity 27, 76–88 (2007).
Altan-Bonnet, G. & Germain, R. N. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 3, e356 (2005).
Chan, C., George, A. J. & Stark, J. Cooperative enhancement of specificity in a lattice of T cell receptors. Proc. Natl Acad. Sci. USA 98, 5758–5763 (2001).
Schamel, W. W., Risueno, R. M., Minguet, S., Ortiz, A. R. & Alarcon, B. A conformation- and avidity-based proofreading mechanism for the TCR–CD3 complex. Trends Immunol. 27, 176–182 (2006).
Germain, R. N. & Stefanova, I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu. Rev. Immunol. 17, 467–522 (1999).
Stefanova, I. et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nature Immunol. 4, 248–254 (2003).
Starr, T. K., Jameson, S. C. & Hogquist, K. A. Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003).
Garcia, K. C., Adams, J. J., Feng, D. & Ely, L. K. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nature Immunol. 10, 143–147 (2009).
Marrack, P., Scott-Browne, J. P., Dai, S., Gapin, L. & Kappler, J. W. Evolutionarily conserved amino acids that control TCR–MHC interaction. Annu. Rev. Immunol. 26, 171–203 (2008).
Rudolph, M. G., Stanfield, R. L. & Wilson, I. A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 24, 419–466 (2006).
Tynan, F. E. et al. T cell receptor recognition of a 'super-bulged' major histocompatibility complex class I-bound peptide. Nature Immunol. 6, 1114–1122 (2005).
Burrows, S. R. et al. Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proc. Natl Acad. Sci. USA 107, 10608–10613 (2010).
Choudhuri, K. & van der Merwe, P. A. Molecular mechanisms involved in T cell receptor triggering. Semin. Immunol. 19, 255–261 (2007).
Valitutti, S., Müller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375, 148–151 (1995).
McKeithan, T. W. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl Acad. Sci. USA 92, 5042–5046 (1995).
Rabinowitz, J. D., Beeson, C., Lyons, D. S., Davis, M. M. & McConnell, H. M. Kinetic discrimination in T-cell activation. Proc. Natl Acad. Sci. USA 93, 1401–1405 (1996).
Cooper, J. A. & Qian, H. A mechanism for SRC kinase-dependent signaling by noncatalytic receptors. Biochemistry 47, 5681–5688 (2008).
Trautmann, A. & Randriamampita, C. Initiation of TCR signalling revisited. Trends Immunol. 24, 425–428 (2003).
Locksley, R. M., Reiner, S. L., Hatam, F., Littman, D. R. & Killeen, N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261, 1448–1451 (1993).
Schilham, M. W. et al. Alloreactive cytotoxic T cells can develop and function in mice lacking both CD4 and CD8. Eur. J. Immunol. 23, 1299–1304 (1993).
Krogsgaard, M. et al. Agonist/endogenous peptide–MHC heterodimers drive T cell activation and sensitivity. Nature 434, 238–243 (2005).
Krogsgaard, M., Juang, J. & Davis, M. M. A role for “self” in T-cell activation. Semin. Immunol. 19, 236–244 (2007).
Juang, J. et al. Peptide–MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J. Exp. Med. 207, 1223–1234 (2010). This study showed that most self peptide–MHC molecules can contribute to negative selection when coupled to an agonist peptide–MHC molecule in peptide–MHC dimers, providing support for the pseudodimer model of TCR triggering, which postulates a critical role for self peptide–MHC molecules.
Alam, S. M. et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity 10, 227–237 (1999).
Reich, Z. et al. Ligand-specific oligomerization of T-cell receptor molecules. Nature 387, 617–620 (1997).
Kuhns, M. S. et al. Evidence for a functional sidedness to the αβTCR. Proc. Natl Acad. Sci. USA 107, 5094–5099 (2010).
Lillemeier, B. F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nature Immunol. 11, 90–96 (2010). A high-resolution imaging study of the distribution of TCR and LAT on the surface of T cells. It suggests that both the TCR and LAT are clustered into small but separate islands that enlarge upon TCR triggering.
Schamel, W. W. et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J. Exp. Med. 202, 493–503 (2005).
Varma, R., Campi, G., Yokosuka, T., Saito, T. & Dustin, M. L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25, 117–127 (2006).
Dunne, P. D. et al. DySCo: quantitating associations of membrane proteins using two-color single-molecule tracking. Biophys. J. 97, L5–L7 (2009).
James, J. R. et al. Single-molecule level analysis of the subunit composition of the T cell receptor on live T cells. Proc. Natl Acad. Sci. USA 104, 17662–17667 (2007).
van der Merwe, P. A., Dunne, P. D., Klenerman, D. & Davis, S. J. Taking T cells beyond the diffraction limit. Nature Immunol. 11, 51–52 (2010).
Yokosuka, T. et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nature Immunol. 6, 1253–1262 (2005).
Campi, G., Varma, R. & Dustin, M. L. Actin and agonist MHC–peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202, 1031–1036 (2005).
Bunnell, S. C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Kjer-Nielsen, L. et al. A structural basis for the selection of dominant αβ T cell receptors in antiviral immunity. Immunity 18, 53–64 (2003).
Beddoe, T. et al. Antigen ligation triggers a conformational change within the constant domain of the αβ T cell receptor. Immunity 30, 777–788 (2009). This paper provides evidence that peptide–MHC binding induces a small but functionally significant conformational change in the distal AB loop of the TCR Cα domain.
Sun, Z.-Y. J. et al. Solution structure of the CD3ɛδ ectodomain and comparison with CD3ɛγ as a basis for modeling T cell receptor topology and signaling. Proc. Natl Acad. Sci. USA 101, 16867–16872 (2004).
Arnett, K. L., Harrison, S. C. & Wiley, D. C. Crystal structure of a human CD3-ɛ/δ dimer in complex with a UCHT1 single-chain antibody fragment. Proc. Natl Acad. Sci. USA 101, 16268–16273 (2004).
Sun, Z.-Y. J., Kim, K. S., Wagner, G. & Reinherz, E. L. Mechanisms contributing to T cell receptor signaling and assembly revealed by the solution structure of an ectodomain fragment of the CD3ɛγ heterodimer. Cell 105, 913–923 (2001).
Kjer-Nielsen, L. et al. Crystal structure of the human T cell receptor CD3ɛγ heterodimer complexed to the therapeutic mAb OKT3. Proc. Natl Acad. Sci. USA 101, 7675–7680 (2004).
Call, M. E. & Wucherpfennig, K. W. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu. Rev. Immunol. 23, 101–125 (2005).
Kuhns, M. S., Davis, M. M. & Garcia, K. C. Deconstructing the form and function of the TCR/CD3 complex. Immunity 24, 133–139 (2006).
Kim, S. T. et al. Distinctive CD3 heterodimeric ectodomain topologies maximize antigen-triggered activation of αβ T cell receptors. J. Immunol. 185, 2951–2959 (2010).
Kuhns, M. S. & Davis, M. M. Disruption of extracellular interactions impairs T cell receptor–CD3 complex stability and signaling. Immunity 26, 357–369 (2007).
Martinez-Martin, N. et al. Cooperativity between T cell receptor complexes revealed by conformational mutants of CD3ɛ. Sci. Signal. 2, ra43 (2009).
Wang, Y. et al. A conserved CXXC motif in CD3ɛ is critical for T cell development and TCR signaling. PLoS Biol. 7, e1000253 (2009).
Mingueneau, M. et al. The proline-rich sequence of CD3ɛ controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nature Immunol. 9, 522–532 (2008).
Wang, H. et al. Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development. EMBO J. 29, 1285–1298 (2010).
Deford-Watts, L. M. et al. The cytoplasmic tail of the T cell receptor CD3ɛ subunit contains a phospholipid-binding motif that regulates T cell functions. J. Immunol. 183, 1055–1064 (2009).
Kuhns, M. S. & Davis, M. M. The safety on the TCR trigger. Cell 135, 594–596 (2008).
Imbert, V. et al. Induction of tyrosine phosphorylation and T-cell activation by vanadate peroxide, an inhibitor of protein tyrosine phosphatases. Biochem. J. 297, 163–173 (1994).
O'Shea, J. J., McVicar, D. W., Bailey, T. L., Burns, C. & Smyth, M. J. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc. Natl Acad. Sci. USA 89, 10306–10310 (1992).
Secrist, J. P., Burns, L. A., Karnitz, L., Koretzky, G. A. & Abraham, R. T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J. Biol. Chem. 268, 5886–5893 (1993).
Fernandes, R. A. et al. What controls T cell receptor phosphorylation? Cell 142, 668–669 (2010).
Gagnon, E. et al. Response multilayered control of T cell receptor phosphorylation. Cell 142, 669–671 (2010).
Ma, Z., Janmey, P. A. & Finkel, T. H. The receptor deformation model of TCR triggering. FASEB J. 22, 1002–1008 (2008).
Choudhuri, A., Kearney, A., Bakker, T. R. & van der Merwe, P. A. Immunology: how do T cells recognize antigen? Curr. Biol. 15, R382–R385 (2005).
Li, Y. C. et al. Cutting edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J. Immunol. 184, 5959–5963 (2010). This study provides evidence that the application of mechanical force on the TCR may induce TCR triggering.
van der Merwe, P. The TCR triggering puzzle. Immunity 14, 665–668 (2001).
Ghendler, Y., Smolyar, A., Chang, H. C. & Reinherz, E. L. One of the CD3ɛ subunits within a T cell receptor complex lies in close proximity to the Cβ FG loop. J. Exp. Med. 187, 1529–1536 (1998).
Geppert, T. D. & Lipsky, P. E. Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3. J. Immunol. 138, 1660–1666 (1987).
Ma, Z., Sharp, K. A., Janmey, P. A. & Finkel, T. H. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 6, e43 (2008).
Choudhuri, K. et al. Peptide–MHC dimensions control proximal kinase–phosphatase balance during T cell activation. J. Biol. Chem. 284, 26096–26105 (2009).
Choudhuri, K., Wiseman, D., Brown, M. H., Gould, K. & van der Merwe, P. A. T cell receptor triggering is critically dependent on the dimensions of its peptide–MHC ligand. Nature 436, 578–582 (2005).
Kim, S. T. et al. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 284, 31028–31037 (2009).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signalling leading to T cell activation results from prolonged T cell receptor occupancy. Role of the T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Davis, M. M. A new trigger for T cells. Cell 110, 285–287 (2002).
Call, M. E., Pyrdol, J., Wiedmann, M. & Wucherpfennig, K. W. The organizing principle in the formation of the T cell receptor–CD3 complex. Cell 111, 967–979 (2002).
Call, M. E. et al. The structure of the ζζ transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell 127, 355–368 (2006).
Tolar, P. & Pierce, S. K. A conformation-induced oligomerization model for B cell receptor microclustering and signaling. Curr. Top. Microbiol. Immunol. 340, 155–169 (2010).
Tolar, P., Hanna, J., Krueger, P. D. & Pierce, S. K. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity 30, 44–55 (2009).
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010). This study showed that a large proportion of LCK in resting T cells is already in the active form and that this does not seem to change significantly upon TCR triggering.
Davis, S. J. & van der Merwe, P. A. The structure and ligand interactions of CD2: implications for T-cell function. Immunol. Today 17, 177–187 (1996).
van der Merwe, P. A., Davis, S. J., Shaw, A. S. & Dustin, M. L. Cytoskeletal polarization and redistribution of cell surface molecules during T cell antigen recognition. Semin. Immunol. 12, 5–21 (2000).
Horejsi, V. Lipid rafts and their roles in T-cell activation. Microbes Infect. 7, 310–316 (2005).
Davis, S. J. & van der Merwe, P. A. The kinetic-segregation model: TCR triggering and beyond. Nature Immunol. 7, 803–809 (2006).
Lin, J. & Weiss, A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J. Cell Biol. 162, 673–682 (2003).
Irles, C. et al. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nature Immunol. 4, 189–197 (2003).
Bluemel, C. et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol. Immunother. 59, 1197–1209 (2010).
James, S. E. et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 180, 7028–7038 (2008).
Munro, S. Lipid rafts: elusive or illusive? Cell 115, 377–388 (2003).
Lingwood, D. & Simons, K. Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010).
Gaus, K., Chklovskaia, E., Fazekas de St. Groth, B., Jessup, W. & Harder, T. Condensation of the plasma membrane at the site of T lymphocyte activation. J. Cell Biol. 171, 121–131 (2005).
Zech, T. et al. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 28, 466–476 (2009).
Harder, T. & Sangani, D. Plasma membrane rafts engaged in T cell signalling: new developments in an old concept. Cell Commun. Signal. 7, 21 (2009).
Glebov, O. O. & Nichols, B. J. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nature Cell Biol. 6, 238–243 (2004).
Hashimoto-Tane, A. et al. T-cell receptor microclusters critical for T-cell activation are formed independently of lipid raft clustering. Mol. Cell. Biol. 30, 3421–3429 (2010).
Pizzo, P. et al. Lipid rafts and T cell receptor signaling: a critical re-evaluation. Eur. J. Immunol. 32, 3082–3091 (2002).
Zhu, M., Shen, S., Liu, Y., Granillo, O. & Zhang, W. Cutting edge: localization of linker for activation of T cells to lipid rafts is not essential in T cell activation and development. J. Immunol. 174, 31–35 (2005).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Huppa, J. B. et al. TCR–peptide–MHC interactions in situ show accelerated kinetics and increased affinity. Nature 463, 963–967 (2010). References 105 and 106 measured the two-dimensional affinity and kinetics of TCR–peptide–MHC interactions for the first time.
Zarnitsyna, V. I. et al. Memory in receptor–ligand-mediated cell adhesion. Proc. Natl Acad. Sci. USA 104, 18037–18042 (2007).
Acknowledgements
We thank our many scientific colleagues and collaborators, including S. Cordoba, H. Zhang, S. Davis, T. Harder, M. Brown, N. Barclay and O. Acuto, for many valuable discussions and insights, and for communicating results before publication. We also thank the referees for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A sequence that is present in the cytoplasmic domains of the invariant chains of various cell-surface immune receptors, such as the T cell receptor–CD3 complex. Following phosphorylation of their tyrosine residue, ITAMs function as docking sites for Src homology 2 (SH2) domain-containing tyrosine kinases and adaptor molecules, thereby facilitating intracellular signalling cascades.
- Serial-triggering model
-
A model that was proposed to account for the observation that small numbers of agonist peptide–MHC complexes seemed to trigger large numbers of T cell receptors (TCRs), and that postulates that a given peptide–MHC complex can serially bind to and trigger multiple TCRs. As it is the number of productive TCR engagements that determines peptide–MHC efficacy, high-affinity peptide–MHC complexes with long half-lives may be less effective. According to this model there is an optimal affinity or half-life for a TCR–peptide–MHC complex.
- Kinetic proof-reading model
-
A model that was proposed to account for the ability of T cells to discriminate between peptide–MHC ligands that have small differences in their affinity or half-life. It postulates that T cell receptor (TCR) triggering requires multiple sequential steps that can only proceed while the TCR is engaged with a peptide–MHC complex and that are completely reversed as soon as the TCR dissociates from the complex.
- Lipid raft
-
An area of the plasma membrane that is rich in cholesterol, glycosphingolipids, glycosylphosphatidylinositol-anchored proteins and several signalling proteins — such as Src family kinases, LAT (linker for activation of T cells) and PAG (protein associated with glycolipid-enriched microdomains).These domains are also known as glycolipid-enriched microdomains (GEMs) and detergent-insoluble glycolipid-enriched membranes (DIGs).
Rights and permissions
About this article
Cite this article
van der Merwe, P., Dushek, O. Mechanisms for T cell receptor triggering. Nat Rev Immunol 11, 47–55 (2011). https://doi.org/10.1038/nri2887
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2887
This article is cited by
-
Multimodal probing of T-cell recognition with hexapod heterostructures
Nature Methods (2024)
-
Cell volume controlled by LRRC8A-formed volume-regulated anion channels fine-tunes T cell activation and function
Nature Communications (2023)
-
Membrane-anchored DNA nanojunctions enable closer antigen-presenting cell–T-cell contact in elevated T-cell receptor triggering
Nature Nanotechnology (2023)
-
Using CombiCells, a platform for titration and combinatorial display of cell surface ligands, to study T-cell antigen sensitivity modulation by accessory receptors
The EMBO Journal (2023)
-
Discrete LAT condensates encode antigen information from single pMHC:TCR binding events
Nature Communications (2022)