Key Points
-
Herpesviruses are a large family of DNA viruses, eight of which can cause diseases in humans, particularly in children and immunocompromised individuals. All herpesviruses have the capacity to cause lytic infection in permissive cells and to establish latent or recurrent infections in other cell types.
-
The innate immune system detects infections using germline-encoded pattern recognition receptors (PRRs). Toll-like receptors (TLRs) are membrane-bound PRRs that detect microorganisms in extracellular and endosomal locations. TLR2, TLR3 and TLR9 are well-described sensors of herpesvirus infections.
-
In the cytoplasm, active innate immune surveillance takes place and is mediated by nucleic acid sensors. Herpesvirus infections are sensed by both RNA and DNA sensing systems, and recent reports suggest a particularly important role for the cytosolic DNA-sensing AIM2-like receptor (ALR) family in intracellular detection of herpesviruses.
-
Given the relatively slow replication cycle of herpesviruses and the establishment of life-long infections, these viruses are highly dependent on efficient immune evasion strategies. It is now known that herpesviruses evade all classes of PRRs (including ALRs), as well as the downstream signalling machinery.
-
Innate immune defence against herpesviruses is highly dependent on the type I interferon system and natural killer cells. Model studies in mice and genetic data from humans have revealed essential roles for both TLRs and intracellular DNA sensors in mounting protective immune responses against herpesviruses.
Abstract
Advances in innate immunity over the past decade have revealed distinct classes of pattern recognition receptors (PRRs) that detect pathogens at the cell surface and in intracellular compartments. This has shed light on how herpesviruses, which are large disease-causing DNA viruses that replicate in the nucleus, are initially recognized during cellular infection. Surprisingly, this involves multiple PRRs both on the cell surface and within endosomes and the cytosol. In this article we describe recent advances in our understanding of innate detection of herpesviruses, how this innate detection translates into anti-herpesvirus host defence, and how the viruses seek to evade this innate detection to establish persistent infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
28 June 2013
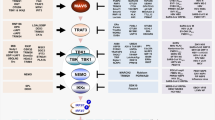
In Figure 3 of the original version of this article, viral RNA was shown to interact with TLR9 and viral DNA was shown to interact with TLR3. This is incorrect and instead viral RNA interacts with TLR3 and viral DNA interacts with TLR9. This has now been corrected online. Nature Reviews Immunology apologizes for this error.
References
Pellett, P. E. & Roizman, B. The Family: Herpesviridae A Brief Introduction in Fields Virology 2479–2500 (Lippincort, Williams & Wilkins, Philadelphia, 2007).
Liu, T., Khanna, K. M., Chen, X., Fink, D. J. & Hendricks, R. L. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191, 1459–1466 (2000).
Liu, T., Khanna, K. M., Carriere, B. N. & Hendricks, R. L. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75, 11178–11184 (2001).
Reusser, P., Riddell, S. R., Meyers, J. D. & Greenberg, P. D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78, 1373–1380 (1991).
Braaten, D. C., Sparks-Thissen, R. L., Kreher, S., Speck, S. H. & Virgin, H. W. An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection. J. Virol. 79, 2573–2583 (2005).
Dupuis, S. et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nature Genet. 33, 388–391 (2003). This paper showed that patients with STAT1 deficiency do not respond to type I IFNs and are susceptible to herpes simplex virus encephalitis.
Salazar-Mather, T. P., Lewis, C. A. & Biron, C. A. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1α delivery to the liver. J. Clin. Invest. 110, 321–330 (2002).
Biron, C. A., Byron, K. S. & Sullivan, J. L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735 (1989).
Rager-Zisman, B., Quan, P. C., Rosner, M., Moller, J. R. & Bloom, B. R. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 138, 884–888 (1987).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Schlee, M. et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Schmidt, A. et al. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl Acad. Sci. USA 106, 12067–12072 (2009).
Pichlmair, A. et al. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 83, 10761–10769 (2009).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007). DAI was the first cytoplasmic DNA sensor to be identified, and was shown to contribute to the type I IFN response to HSV-1 infection.
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature Immunol. 10, 266–272 (2009).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Roberts, T. L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nature Immunol. 10, 1065–1072 (2009).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Yang, P. et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a β-catenin-dependent pathway. Nature Immunol. 11, 487–494 (2010).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nature Immunol. 11, 997–1004 (2010). This paper identifies IFI16 as an intracellular sensor of DNA, and shows that it is essential for the IFN and cytokine response to HSV-1 infection. The work is also the first to demonstrate the presence free viral genomic DNA in the cytoplasm during infection.
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nature Immunol. 11, 63–69 (2010).
Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature Rev. Immunol. 6, 644–658 (2006).
Gugliesi, F. et al. The proapoptotic activity of the interferon-inducible gene IFI16 provides new insights into its etiopathogenetic role in autoimmunity. J. Autoimmun. 35, 114–123 (2010).
Chandran, B. Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J. Virol. 84, 2188–2199 (2010).
Melroe, G. T., DeLuca, N. A. & Knipe, D. M. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78, 8411–8420 (2004).
Cristea, I. M. et al. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J. Virol. 84, 7803–7814 (2010).
Cotter, C. R. et al. The virion host shut-off (vhs) protein blocks a TLR-independent pathway of herpes simplex virus type 1 recognition in human and mouse dendritic cells. PLoS ONE 5, e8684 (2010).
Rebsamen, M. et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-κB. EMBO Rep. 10, 916–922 (2009).
Wu, L. et al. Epstein-Barr virus LF2: an antagonist to type I interferon. J. Virol. 83, 1140–1146 (2009).
van Lint, A. L. et al. Herpes simplex virus immediate-early ICP0 protein inhibits TLR2-dependent inflammatory responses and NF-κB signaling. J. Virol. 84, 10802–10811 (2010).
Orvedahl, A. et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1, 23–35 (2007).
Melchjorsen, J., Siren, J., Julkunen, I., Paludan, S. R. & Matikainen, S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-κB and IRF-3. J. Gen.Virol. 87, 1099–1108 (2006).
Verpooten, D., Ma, Y., Hou, S., Yan, Z. & He, B. Control of TANK-binding kinase 1-mediated signaling by the γ134.5 protein of herpes simplex virus 1. J. Biol. Chem. 284, 1097–1105 (2009).
Jong, J. E., Park, J., Kim, S. & Seo, T. Kaposi's sarcoma-associated herpesvirus viral protein kinase interacts with RNA helicase A and regulates host gene expression. J. Microbiol. 48, 206–212 (2010).
Zhu, F. X., King, S. M., Smith, E. J., Levy, D. E. & Yuan, Y. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl Acad. Sci. USA 99, 5573–5578 (2002).
Jaworska, J., Gravel, A., Fink, K., Grandvaux, N. & Flamand, L. Inhibition of transcription of the β interferon gene by the human herpesvirus 6 immediate-early 1 protein. J. Virol. 81, 5737–5748 (2007).
Lubyova, B. & Pitha, P. M. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74, 8194–8201 (2000).
Taylor, R. T. & Bresnahan, W. A. Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor α-induced NFκB-dependent gene expression. J. Virol. 80, 10763–10771 (2006).
Cloutier, N. & Flamand, L. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits interferon (IFN) β expression by competing with IFN regulatory factor-3 for binding to IFNB promoter. J. Biol. Chem. 285, 7208–7221 (2010).
Sen, N. et al. Varicella zoster virus immediate early protein 62 blocks IRF3 phosphorylation at key serine residues: a novel mechanism of IRF3 inhibition among herpesviruses. J. Virol. 84, 9240–9253 (2010).
Akhtar, J. & Shukla, D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 276, 7228–7236 (2009).
Subramanian, R. P. & Geraghty, R. J. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl Acad. Sci. USA 104, 2903–2908 (2007).
Atanasiu, D., Saw, W. T., Cohen, G. H. & Eisenberg, R. J. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 84, 12292–12299 (2010).
Boehme, K. W., Guerrero, M. & Compton, T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177, 7094–7102 (2006).
Compton, T. et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77, 4588–4596 (2003). This paper was the first to identify a TLR involved in the recognition of a herpesvirus.
Kurt-Jones, E. A. et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl Acad. Sci. USA 101, 1315–1320 (2004).
Sorensen, L. N. et al. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 181, 8604–8612 (2008).
Wang, J. P. et al. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J. Virol. 79, 12658–12666 (2005).
Gaudreault, E., Fiola, S., Olivier, M. & Gosselin, J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J. Virol. 81, 8016–8024 (2007).
Sato, A., Linehan, M. M. & Iwasaki, A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl Acad. Sci. USA 103, 17343–17348 (2006).
Reske, A., Pollara, G., Krummenacher, C., Katz, D. R. & Chain, B. M. Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J. Immunol. 180, 7525–7536 (2008).
Barbalat, R., Lau, L., Locksley, R. M. & Barton, G. M. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nature Immunol. 10, 1200–1207 (2009).
Szomolanyi-Tsuda, E., Liang, X., Welsh, R. M., Kurt-Jones, E. A. & Finberg, R. W. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J. Virol. 80, 4286–4291 (2006).
Bochud, P. Y., Magaret, A. S., Koelle, D. M., Aderem, A. & Wald, A. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus type 2 infection. J. Infect. Dis. 196, 505–509 (2007).
Kijpittayarit, S., Eid, A. J., Brown, R. A., Paya, C. V. & Razonable, R. R. Relationship between Toll-like receptor 2 polymorphism and cytomegalovirus disease after liver transplantation. Clin. Infect. Dis. 44, 1315–1320 (2007).
Ahmad-Nejad, P. et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32, 1958–1968 (2002).
Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003). This paper was the first to identify a TLR involved in the recognition of herpesvirus nucleic acids.
Krug, A. et al. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103, 1433–1437 (2004).
Varani, S. et al. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J. Immunol. 179, 7767–7776 (2007).
Lim, W. H., Kireta, S., Russ, G. R. & Coates, P. T. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood 109, 1043–1050 (2007).
Fiola, S., Gosselin, D., Takada, K. & Gosselin, J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 185, 3620–3631 (2010).
Tabeta, K. et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl Acad. Sci. USA 101, 3516–3521 (2004).
Krug, A. et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21, 107–119 (2004).
Guggemoos, S. et al. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J. Immunol. 180, 438–443 (2008).
Yasuda, K. et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J. Immunol. 183, 3109–3117 (2009).
Haas, T. et al. The DNA sugar backbone 2′ deoxyribose determines Toll-like receptor 9 activation. Immunity 28, 315–323 (2008).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Delale, T. et al. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-α release and initiation of immune responses in vivo. J. Immunol. 175, 6723–6732 (2005).
Rasmussen, S. B. et al. Type I IFN production during HSV infection is controlled by cell-type specific viral recognition by TLR9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 81, 13315–13324 (2007).
Sarangi, P. P., Kim, B., Kurt-Jones, E. & Rouse, B. T. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J. Virol. 81, 11128–11138 (2007).
Lund, J. M., Linehan, M. M., Iijima, N. & Iwasaki, A. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177, 7510–7514 (2006).
Wuest, T. et al. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J. Neuroimmunol. 179, 46–52 (2006).
Zucchini, N. et al. Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J. Immunol. 180, 5799–5803 (2008).
Hokeness-Antonelli, K. L., Crane, M. J., Dragoi, A. M., Chu, W. M. & Salazar-Mather, T. P. IFN-αβ-mediated inflammatory responses and antiviral defense in liver is TLR9-independent but MyD88-dependent during murine cytomegalovirus infection. J. Immunol. 179, 6176–6183 (2007).
Ku, C. L. et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 204, 2407–2422 (2007).
Weber, F., Wagner, V., Rasmussen, S. B., Hartmann, R. & Paludan, S. R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80, 5059–5064 (2006). This report showed that double-stranded RNA accumulates in the cytoplasm of permissive cells infected with HSV-1.
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Iwakiri, D. et al. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 206, 2091–2099 (2009).
Miettinen, M., Sareneva, T., Julkunen, I. & Matikainen, S. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2, 349–355 (2001).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Davey, G. M. et al. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J. Immunol. 184, 2243–2246 (2010).
Zhang, S. Y. et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527 (2007). This study showed that patients with TLR3 deficiency exhibit an impaired IFN response to HSV-1 infection in vitro and enhanced susceptibility to herpes simplex virus encephalitis.
Ranjith-Kumar, C. T. et al. Effects of single nucleotide polymorphisms on Toll-like receptor 3 activity and expression in cultured cells. J. Biol. Chem. 282, 17696–17705 (2007).
Willmann, O., Ahmad-Nejad, P., Neumaier, M., Hennerici, M. G. & Fatar, M. Toll-like receptor 3 immune deficiency may be causative for HSV-2-associated mollaret meningitis. Eur. Neurol. 63, 249–251 (2010).
Sciortino, M. T., Suzuki, M., Taddeo, B. & Roizman, B. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J. Virol. 75, 8105–8116 (2001).
Bresnahan, W. A. & Shenk, T. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288, 2373–2376 (2000).
Malmgaard, L., Melchjorsen, J., Bowie, A. G., Mogensen, S. C. & Paludan, S. R. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173, 6890–6898 (2004).
Samanta, M., Iwakiri, D., Kanda, T., Imaizumi, T. & Takada, K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25, 4207–4214 (2006).
Samanta, M., Iwakiri, D. & Takada, K. Epstein-Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene 27, 4150–4160 (2008).
Melchjorsen, J. et al. Early innate recognition of herpes simplex virus in human primary macrophages is mediated via the MDA5/MAVS-dependent and MDA5/MAVS/RNA polymerase III-independent pathways. J. Virol. 84, 11350–11358 (2010).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004).
Ishii, K. J. et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nature Immunol. 7, 40–48 (2006). This paper was the first to report that herpesvirus (HSV and HCMV) DNA has the capacity to stimulate innate immune responses through cytosolic PRRs.
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Defilippis, V. R. et al. Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B. J. Virol. 84, 8913–8925 (2010).
DeFilippis, V. R., Alvarado, D., Sali, T., Rothenburg, S. & Fruh, K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J. Virol. 84, 585–598 (2010).
Cheng, G., Zhong, J., Chung, J. & Chisari, F. V. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl Acad. Sci. USA 104, 9035–9040 (2007).
Choi, M. K. et al. A selective contribution of the RIG-I-like receptor pathway to type I interferon responses activated by cytosolic DNA. Proc. Natl Acad. Sci. USA 106, 17870–17875 (2009).
Kim, T. et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl Acad. Sci. USA 107, 15181–15186 (2010).
Yan, H. et al. RPA nucleic acid-binding properties of IFI16-HIN200. Biochim. Biophys. Acta 1784, 1087–1097 (2008).
Mondini, M. et al. The interferon-inducible HIN-200 gene family in apoptosis and inflammation: implication for autoimmunity. Autoimmunity 43, 226–231 (2010).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature Immunol. 11, 395–402 (2010). This work is the first to demonstrate an essential role for a cytoplasmic DNA sensor in the innate immune response to a herpesvirus (MCMV).
Hertel, L. et al. The interferon-inducible 204 gene, a member of the Ifi 200 family, is not involved in the antiviral state induction by IFN-α, but is required by the mouse cytomegalovirus for its replication. Virology 262, 1–8 (1999).
Rehwinkel, J. et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 140, 397–408 (2010).
Lee, H. K., Lund, J. M., Ramanathan, B., Mizushima, N. & Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315, 1398–1401 (2007).
Melchjorsen, J. & Paludan, S. R. Induction of RANTES/CCL5 by herpes simplex virus is regulated by nuclear factor κB and interferon regulatory factor 3. J. Gen. Virol. 84, 2491–2495 (2003).
Delboy, M. G., Roller, D. G. & Nicola, A. V. Cellular proteasome activity facilitates herpes simplex virus entry at a postpenetration step. J. Virol. 82, 3381–3390 (2008).
Soulat, D. et al. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J. 27, 2135–2146 (2008).
Schroder, M., Baran, M. & Bowie, A. G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKɛ-mediated IRF activation. EMBO J. 27, 2147–2157 (2008).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009). References 113 and 114 identify and describe STING as an essential adaptor in intracellular DNA signalling, and reveal the importance of subcellular localization in innate immune recognition and signalling.
Saitoh, T. et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl Acad. Sci. USA 106, 20842–20846 (2009).
Smiley, J. R. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78, 1063–1068 (2004).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nature Immunol. 7, 131–137 (2006).
Abate, D. A., Watanabe, S. & Mocarski, E. S. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 78, 10995–11006 (2004).
Browne, E. P. & Shenk, T. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl Acad. Sci. USA 100, 11439–11444 (2003).
McGregor, A., Liu, F. & Schleiss, M. R. Molecular, biological, and in vivo characterization of the guinea pig cytomegalovirus (CMV) homologs of the human CMV matrix proteins pp71 (UL82) and pp65 (UL83). J. Virol. 78, 9872–9889 (2004).
Leib, D. A. et al. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189, 663–672 (1999).
Saira, K., Zhou, Y. & Jones, C. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces degradation of interferon response factor 3 and, consequently, inhibits β interferon promoter activity. J. Virol. 81, 3077–3086 (2007).
Paladino, P., Collins, S. E. & Mossman, K. L. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS ONE 5, e10428 (2010).
Melroe, G. T., Silva, L., Schaffer, P. A. & Knipe, D. M. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-β induction. Virology 360, 305–321 (2007).
Joo, C. H. et al. Inhibition of interferon regulatory factor 7 (IRF7)-mediated interferon signal transduction by the Kaposi's sarcoma-associated herpesvirus viral IRF homolog vIRF3. J. Virol. 81, 8282–8292 (2007).
Wies, E. et al. The Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J. Biol. Chem. 284, 8525–8538 (2009).
Donaghy, H. et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J. Virol. 83, 1952–1961 (2009).
Sainz, B., Jr & Halford, W. P. α/β interferon and γ interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76, 11541–11550 (2002).
Gargano, L. M., Moser, J. M. & Speck, S. H. Role for MyD88 signaling in murine gammaherpesvirus 68 latency. J. Virol. 82, 3853–3863 (2008).
Gregory, S. M. et al. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc. Natl Acad. Sci. USA 106, 11725–11730 (2009).
Gargano, L. M., Forrest, J. C. & Speck, S. H. Signaling through Toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J. Virol. 83, 1474–1482 (2009).
Kristensen, H. et al. Extracellular 2′-5′ oligoadenylate synthetase stimulates RNase L-independent antiviral activity: a novel mechanism of virus-induced innate immunity. J. Virol. 84, 11898–11904 (2010).
Haahr, S. & Hollsberg, P. Multiple sclerosis is linked to Epstein-Barr virus infection. Rev. Med. Virol. 16, 297–310 (2006).
Toussirot, E. & Roudier, J. Epstein-Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 22, 883–896 (2008).
Leadbetter, E. A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Kimkong, I., Avihingsanon, Y. & Hirankarn, N. Expression profile of HIN200 in leukocytes and renal biopsy of SLE patients by real-time RT-PCR. Lupus 18, 1066–1072 (2009).
Mondini, M. et al. Role of the interferon-inducible gene IFI16 in the etiopathogenesis of systemic autoimmune disorders. Ann. NY Acad. Sci. 1110, 47–56 (2007).
Casrouge, A. et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314, 308–312 (2006).
Diego, R. P. et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33, 400–411 (2010).
Ariza, M. E., Glaser, R., Kaumaya, P. T., Jones, C. & Williams, M. V. The EBV-encoded dUTPase activates NF-κB through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 182, 851–859 (2009).
Acknowledgements
This work was supported by grants awarded to S.R.P. from the Danish Medical Research Council (09-072,636), the Lundbeck Foundation (R34-A3855), Velux Fonden, Kathrine og Vigo Skovgaards Fond and Elvira og Rasmus Riisforts almenvelgørende Fond; by grants awarded to K.A.F. from the US National Institutes of Health (AI067497, AI64349, AI083713 and AI079293); and by grants to A.G.B. from Science Foundation Ireland. K.A.H. was supported by a Marie Curie Incoming International Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- Lytic infection
-
Viral replication in host cells either causes cellular death and lysis (lytic infection) or is compatible with cell survival (persistent infection).
- Permissive cells
-
Cells that support the replication of a given virus. Permissiveness is often determined by the expression of specific components in the cells and/or the ability of viruses to circumvent host defence mechanisms.
- Latency
-
A state in which viruses lie dormant in infected cells with no detectable viral replication. From the latent state, viruses can become activated to initiate productive replication.
- Pathogen-associated molecular patterns
-
(PAMPs). Evolutionarily conserved molecular structures that are recognized by pattern recognition receptors. PAMPs are either specific for whole classes of pathogens or used by both microorganism and host but present in abnormal locations.
- IFN-stimulated genes
-
(ISGs). Genes that are induced by interferons (IFNs) through their IFN-stimulated response element (ISRE), which is bound by the IFN-activated transcription factor ISGF3. A subgroup of ISGs is induced by pattern recognition receptor signalling, through transcription factors of the IRF family.
- Cross-presentation
-
The ability of certain antigen-presenting cells (APCs) to load peptides that are derived from exogenous antigens onto MHC class I molecules. This property is atypical, because most cells exclusively present peptides from their endogenous proteins on MHC class I molecules. Cross-presentation is essential for the initiation of immune responses to viruses that do not infect APCs.
- Autophagy
-
A catabolic process in which cells degrade cytosolic content, including organelles, through the lysosomal machinery.
- Pyrin and HIN domain-containing protein family
-
A family of proteins with both pyrin and HIN domains. Pyrin domains mediate pyrin–pyrin homotypic interactions and stimulate signal transduction, whereas HIN domains bind DNA.
- Neurovirulence factor
-
A factor that is essential for the ability of a pathogen to be pathogenic in the nervous system. Herpes simplex virus and rabies virus are neurovirulent viruses.
- Tegument
-
An amorphous layer of proteins that lines the space between the lipid membrane and the nucleocapsid of herpesviruses. The tegument proteins generally support viral replication and evasion of immune responses.
- 2′-5′-oligoadenylate synthetase
-
A family of interferon- and virus-inducible enzymes that catalyses the formation 2′-5′ oligomers of adenosine to stimulate antiviral activities that are dependent and independent of RNase L.
Rights and permissions
About this article
Cite this article
Paludan, S., Bowie, A., Horan, K. et al. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 11, 143–154 (2011). https://doi.org/10.1038/nri2937
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2937
This article is cited by
-
The impact of HLA polymorphism on herpesvirus infection and disease
Immunogenetics (2023)
-
Potential role of EBV and Toll-like receptor 9 ligand in patients with systemic lupus erythematosus
Immunologic Research (2023)
-
Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA
Nature (2022)
-
Co-infection of porcine deltacoronavirus and porcine epidemic diarrhea virus induces early TRAF6-mediated NF-κB and IRF7 signaling pathways through TLRs
Scientific Reports (2022)
-
Risk of herpes zoster in psoriasis patients receiving systemic therapies: a nationwide population-based cohort study
Scientific Reports (2021)