Key Points
-
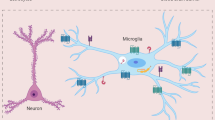
Specialized endothelial cells in the central nervous system (CNS) limit cellular and ionic movement into the brain parenchyma and act as a critical component of the blood–cerebral spinal fluid and blood–brain barriers. In certain anatomical locations, macrophages, microglia and astrocytes are juxtaposed to CNS blood vessels, and this positions these cells to present foreign antigens and/or provide additional barrier support. Innate immune cells such as macrophages and dendritic cells are also found in the meninges and choroid plexus, enabling surveillance of fluid spaces.
-
Viruses use several different strategies to bypass protective barriers and access the CNS. These strategies include haematological entry mechanisms, such as direct infection of vascular endothelium or travelling in immune cells across CNS barriers through a 'Trojan horse' mechanism. Viruses can also access peripheral nerves that reside outside the protective CNS barriers.
-
Immune responses to neurotropic viruses can promote viral clearance or latency, but sometimes give rise to pathology and disease. HIV persists in CNS myeloid cells (macrophages and microglia), giving rise to chronic innate and adaptive immune responses. This pro-inflammatory milieu can eventually cause neuronal damage and dementia. By contrast, herpes simplex virus latency in sensory ganglion neurons is maintained without injury, in part by innate cytokines and virus-specific T cells.
-
Two-photon laser scanning microscopy (TPLSM) is a microscopic technique that can be used to monitor the dynamics of immune responses to neurotropic viruses in real time. When conducting TPLSM experiments, the tissue preparation must be carefully considered because certain preparations can give rise to injury responses that confound data interpretation. Craniotomies and acute brain slices induce considerable tissue damage, whereas skull thinning opens a window for TPLSM imaging without brain injury.
-
Intravital TPLSM imaging of innate immune sentinels, such as dendritic cells, macrophages and microglia, can provide novel insights into their function within the normal and inflamed brain. Studies have revealed that microglia, for example, are highly dynamic under steady-state conditions and rapidly redirect their cellular processes to engulf debris following tissue injury.
-
Intravital imaging of CNS-infiltrating leukocytes during fatal viral meningitis has revealed that recruitment of myelomonocytic cells by virus-specific cytotoxic lymphocytes causes severe vascular injury and the rapid onset of convulsive seizures. Future imaging studies of CNS inflammatory responses following viral infection are required to determine how the immune system operates during states of viral clearance, latency and persistence.
Abstract
Viral infections are a major cause of human disease. Although most viruses replicate in peripheral tissues, some have developed unique strategies to move into the nervous system, where they establish acute or persistent infections. Viral infections in the central nervous system (CNS) can alter homeostasis, induce neurological dysfunction and result in serious, potentially life-threatening inflammatory diseases. This Review focuses on the strategies used by neurotropic viruses to cross the barrier systems of the CNS and on how the immune system detects and responds to viral infections in the CNS. A special emphasis is placed on immune surveillance of persistent and latent viral infections and on recent insights gained from imaging both protective and pathogenic antiviral immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). This study demonstrated that microglial cells are derived from primitive myeloid precursors rather than haematopoietic cells.
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). This study used TPLSM to show that microglial processes are highly dynamic and continually scan the naive brain parenchyma.
Bulloch, K. et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J. Compar. Neurol. 508, 687–710 (2008).
Chinnery, H. R., Ruitenberg, M. J. & McMenamin, P. G. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J. Neuropathol. Exp. Neurol. 69, 896–909 (2010).
Tyler, K. L. Emerging viral infections of the central nervous system: part 2. Arch. Neurol. 66, 1065–1074 (2009).
Tyler, K. L. Emerging viral infections of the central nervous system: part 1. Arch. Neurol. 66, 939–948 (2009).
Bechmann, I., Galea, I. & Perry, V. H. What is the blood–brain barrier (not)? Trends Immunol. 28, 5–11 (2007).
Kutcher, M. E. & Herman, I. M. The pericyte: cellular regulator of microvascular blood flow. Microvasc. Res. 77, 235–246 (2009).
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Grossmann, R. et al. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 37, 229–240 (2002).
Hickey, W. F. & Kimura, H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239, 290–292 (1988).
Prodinger, C. et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 121, 445–458 (2011).
Bartholomaus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009).
Mathiisen, T. M., Lehre, K. P., Danbolt, N. C. & Ottersen, O. P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103 (2010).
Chapagain, M. L., Verma, S., Mercier, F., Yanagihara, R. & Nerurkar, V. R. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology 364, 55–63 (2007).
Coyne, C. B., Kim, K. S. & Bergelson, J. M. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. EMBO J. 26, 4016–4028 (2007).
Casiraghi, C., Dorovini-Zis, K. & Horwitz, M. S. Epstein-Barr virus infection of human brain microvessel endothelial cells: a novel role in multiple sclerosis. J. Neuroimmunol. 230, 173–177 (2010).
Gralinski, L. E., Ashley, S. L., Dixon, S. D. & Spindler, K. R. Mouse adenovirus type 1-induced breakdown of the blood–brain barrier. J. Virol. 83, 9398–9410 (2009).
Afonso, P. V. et al. Alteration of blood–brain barrier integrity by retroviral infection. PLoS Pathog. 4, e1000205 (2008).
Verma, S., Kumar, M., Gurjav, U., Lum, S. & Nerurkar, V. R. Reversal of West Nile virus-induced blood–brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 397, 130–138 (2010).
Antar, A. A. et al. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 5, 59–71 (2009).
Alexaki, A. & Wigdahl, B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 4, e1000215 (2008).
Clay, C. C. et al. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J. Virol. 81, 12040–12048 (2007).
Ancuta, P., Wang, J. & Gabuzda, D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J. Leukoc. Biol. 80, 1156–1164 (2006).
Roberts, T. K., Buckner, C. M. & Berman, J. W. Leukocyte transmigration across the blood–brain barrier: perspectives on neuroAIDS. Front. Biosci. 15, 478–536 (2010).
Monaco, M. C., Atwood, W. J., Gravell, M., Tornatore, C. S. & Major, E. O. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J. Virol. 70, 7004–7012 (1996).
Chapagain, M. L. & Nerurkar, V. R. Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood–brain barrier. J. Infect. Dis. 202, 184–191 (2010).
Salinas, S., Schiavo, G. & Kremer, E. J. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nature Rev. Microbiol. 8, 645–655 (2010).
Diefenbach, R. J., Miranda-Saksena, M., Douglas, M. W. & Cunningham, A. L. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 18, 35–51 (2008).
Mata, M., Zhang, M., Hu, X. & Fink, D. J. HveC (nectin-1) is expressed at high levels in sensory neurons, but not in motor neurons, of the rat peripheral nervous system. J. Neurovirol. 7, 476–480 (2001).
Curanovic, D. & Enquist, L. Directional transneuronal spread of α-herpesvirus infection. Future Virol. 4, 591–603 (2009).
Mori, I., Nishiyama, Y., Yokochi, T. & Kimura, Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 11, 129–137 (2005).
Samuel, M. A., Wang, H., Siddharthan, V., Morrey, J. D. & Diamond, M. S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl Acad. Sci. USA 104, 17140–17145 (2007).
Roussarie, J. P., Ruffie, C., Edgar, J. M., Griffiths, I. & Brahic, M. Axon myelin transfer of a non-enveloped virus. PLoS ONE 2, e1331 (2007).
Dietzschold, B., Li, J., Faber, M. & Schnell, M. Concepts in the pathogenesis of rabies. Future Virol. 3, 481–490 (2008).
Young, V. A. & Rall, G. F. Making it to the synapse: measles virus spread in and among neurons. Curr. Top. Microbiol. Immunol. 330, 3–30 (2009).
Bajramovic, J. J. et al. Borna disease virus glycoprotein is required for viral dissemination in neurons. J. Virol. 77, 12222–12231 (2003).
de la Torre, J. C. Bornavirus and the brain. J. Infect. Dis. 186, S241–S247 (2002).
Hsieh, M. J., White, P. J. & Pouton, C. W. Interaction of viruses with host cell molecular motors. Curr. Opin. Biotechnol. 21, 633–639 (2010).
Iannacone, M. et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 (2010). This seminal study showed that subcapsular sinus macrophages in draining lymph nodes can act as 'gatekeepers' to prevent neurotropic viruses from accessing peripheral nerves and entering the CNS.
De Regge, N. et al. α-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 174, 267–275 (2006).
Knipe, D. M. & Cliffe, A. Chromatin control of herpes simplex virus lytic and latent infection. Nature Rev. Microbiol. 6, 211–221 (2008).
Conrady, C. D., Drevets, D. A. & Carr, D. J. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing? J. Neuroimmunol. 220, 1–9 (2010).
Perez de Diego, R. et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33, 400–411 (2010).
Zhou, Y. et al. Activation of Toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells. J. Neurosci. Res. 87, 2916–2925 (2009).
Samuel, C. E. Antiviral actions of interferons. Clin. Microbiol. Rev. 14, 778–809 (2001).
De Regge, N., Van Opdenbosch, N., Nauwynck, H. J., Efstathiou, S. & Favoreel, H. W. Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro. PLoS ONE 5, e13076 (2010).
Sobol, P. T. & Mossman, K. L. ICP0 prevents RNase L-independent rRNA cleavage in herpes simplex virus type 1-infected cells. J. Virol. 80, 218–225 (2006).
Harle, P., Sainz, B. Jr, Carr, D. J. & Halford, W. P. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-α/β. Virology 293, 295–304 (2002).
Khanna, K. M., Lepisto, A. J., Decman, V. & Hendricks, R. L. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16, 463–469 (2004).
Simmons, A. & Tscharke, D. C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175, 1337–1344 (1992).
Khanna, K. M., Bonneau, R. H., Kinchington, P. R. & Hendricks, R. L. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18, 593–603 (2003).
Orr, M. T., Mathis, M. A., Lagunoff, M., Sacks, J. A. & Wilson, C. B. CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I. Cell Host Microbe 2, 172–180 (2007). This study definitively established the importance of MHC class I molecules in controlling HSV-1 latency in sensory neurons.
Sheridan, B. S., Cherpes, T. L., Urban, J., Kalinski, P. & Hendricks, R. L. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J. Virol. 83, 2237–2245 (2009).
Wakim, L. M., Gebhardt, T., Heath, W. R. & Carbone, F. R. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J. Immunol. 181, 5837–5841 (2008).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 10, 524–530 (2009). This study demonstrated that immune responses mediated by local tissue-resident CD8+ T cells are important in controlling HSV infection.
Theil, D. et al. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163, 2179–2184 (2003).
Hufner, K. et al. Latency of α-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J. Neuropathol. Exp. Neurol. 65, 1022–1030 (2006).
Verjans, G. M. et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl Acad. Sci. USA 104, 3496–3501 (2007).
Liu, T., Khanna, K. M., Chen, X., Fink, D. J. & Hendricks, R. L. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191, 1459–1466 (2000).
Decman, V., Freeman, M. L., Kinchington, P. R. & Hendricks, R. L. Immune control of HSV-1 latency. Viral Immunol. 18, 466–473 (2005).
Frank, G. M. et al. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J. Immunol. 184, 277–286 (2010).
Wakim, L. M., Waithman, J., van Rooijen, N., Heath, W. R. & Carbone, F. R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Divito, S., Cherpes, T. L. & Hendricks, R. L. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol. Res. 36, 119–126 (2006).
Derfuss, T. et al. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol. 17, 389–398 (2007).
Mandal, M., Bandyopadhyay, D., Goepfert, T. M. & Kumar, R. Interferon-induces expression of cyclin-dependent kinase-inhibitors p21WAF1 and p27Kip1 that prevent activation of cyclin-dependent kinase by CDK-activating kinase (CAK). Oncogene 16, 217–225 (1998).
Liu, T., Khanna, K. M., Carriere, B. N. & Hendricks, R. L. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75, 11178–11184 (2001).
Knickelbein, J. E. et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322, 268–271 (2008). This study revealed an interesting mechanism by which CTLs use lytic granules to non-cytopathically control HSV-1 in latently infected neurons.
Jiang, X. et al. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J. Virol. 85, 2325–2332 (2011).
Alexaki, A., Liu, Y. & Wigdahl, B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr. HIV Res. 6, 388–400 (2008).
Yadav, A. & Collman, R. G. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J. Neuroimmune Pharmacol. 4, 430–447 (2009).
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 12, 1365–1371 (2006).
Witwer, K. W. et al. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS ONE 4, e8129 (2009).
Barber, S. A. et al. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus–macaque model. J. Infect. Dis. 193, 963–970 (2006).
Barber, S. A., Herbst, D. S., Bullock, B. T., Gama, L. & Clements, J. E. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J. Neurovirol. 10 (Suppl. 1), 15–20 (2004).
Ravimohan, S., Gama, L., Barber, S. A. & Clements, J. E. Regulation of SIVmac239 basal long terminal repeat activity and viral replication in macrophages: functional roles of two CCAAT/enhancer-binding protein β sites in activation and interferon β-mediated suppression. J. Biol. Chem. 285, 2258–2273 (2010).
Witwer, K. W., Sisk, J. M., Gama, L. & Clements, J. E. MicroRNA regulation of IFN-β protein expression: rapid and sensitive modulation of the innate immune response. J. Immunol. 184, 2369–2376 (2010).
Akhtar, L. N. et al. Suppressor of cytokine signaling 3 inhibits antiviral IFN-β signaling to enhance HIV-1 replication in macrophages. J. Immunol. 185, 2393–2404 (2010).
Mankowski, J. L., Clements, J. E. & Zink, M. C. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J. Infect. Dis. 186, S199–S208 (2002).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Jin, X. et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189, 991–998 (1999).
Fox, H. S. Virus–host interaction in the simian immunodeficiency virus-infected brain. J. Neurovirol. 14, 286–291 (2008).
Kim, W. K. et al. Identification of T lymphocytes in simian immunodeficiency virus encephalitis: distribution of CD8+ T cells in association with central nervous system vessels and virus. J. Neurovirol. 10, 315–325 (2004).
Roberts, E. S. et al. Host response and dysfunction in the CNS during chronic simian immunodeficiency virus infection. J. Neurosci. 26, 4577–4585 (2006).
Sadagopal, S. et al. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J. Virol. 82, 10418–10428 (2008).
McCrossan, M. et al. An immune control model for viral replication in the CNS during presymptomatic HIV infection. Brain 129, 503–516 (2006).
Cosenza, M. A., Zhao, M. L., Si, Q. & Lee, S. C. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 12, 442–455 (2002).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Germain, R. N., Miller, M. J., Dustin, M. L. & Nussenzweig, M. C. Dynamic imaging of the immune system: progress, pitfalls and promise. Nature Rev. Immunol. 6, 497–507 (2006).
Zinselmeyer, B. H. et al. Chapter 16. Two-photon microscopy and multidimensional analysis of cell dynamics. Methods Enzymol. 461, 349–378 (2009).
Kang, S. S. & McGavern, D. B. Inflammation on the mind: visualizing immunity in the central nervous system. Curr. Top. Microbiol. Immunol. 334, 227–263 (2009).
Yang, G., Pan, F., Parkhurst, C. N., Grutzendler, J. & Gan, W. B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nature Protoc. 5, 201–208 (2010).
Xu, H. T., Pan, F., Yang, G. & Gan, W. B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nature Neurosci. 10, 549–551 (2007). This important study demonstrated how tissue preparation can influence the results of intravital two-photon imaging studies in the CNS.
Mainen, Z. F. et al. Two-photon imaging in living brain slices. Methods 18, 231–239 (1999).
Wilson, E. H. et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30, 300–311 (2009).
Schaeffer, M. et al. Dynamic imaging of T cell–parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J. Immunol. 182, 6379–6393 (2009).
Bajenoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Brockhaus, J., Moller, T. & Kettenmann, H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia 16, 81–90 (1996).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci. 8, 752–758 (2005). This study demonstrated that extracellular ATP regulates microglial branch dynamics and mediates their rapid response to CNS injury.
Haynes, S. E. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nature Neurosci. 9, 1512–1519 (2006).
Kim, J. V. & Dustin, M. L. Innate response to focal necrotic injury inside the blood–brain barrier. J. Immunol. 177, 5269–5277 (2006).
Vilela, M. C. et al. Traffic of leukocytes in the central nervous system is associated with chemokine up-regulation in a severe model of herpes simplex encephalitis: an intravital microscopy study. Neurosci. Lett. 445, 18–22 (2008).
Kim, J. V., Kang, S. S., Dustin, M. L. & McGavern, D. B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195 (2009). This study uncovered a novel mechanism in the pathogenesis of viral meningitis by demonstrating that this classic CTL-dependent disease depends on the recruitment of pathogenic innate immune cells for vascular breakdown and induction of fatal convulsive seizures.
Kang, S. S. & McGavern, D. B. Lymphocytic choriomeningitis infection of the central nervous system. Front. Biosci. 13, 4529–4543 (2008).
McGavern, D. B., Christen, U. & Oldstone, M. B. Molecular anatomy of antigen-specific CD8+ T cell engagement and synapse formation in vivo. Nature Immunol. 3, 918–925 (2002).
Fung-Leung, W. P., Kundig, T. M., Zinkernagel, R. M. & Mak, T. W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J. Exp. Med. 174, 1425–1429 (1991).
Kang, S. S. & McGavern, D. B. Microbial induction of vascular pathology in the CNS. J. Neuroimmune Pharmacol. 5, 370–386 (2010).
Major, E. O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 61, 35–47 (2010).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353, 375–381 (2005). This study was the first to document JC virus-induced PML in a patient treated with the immunosuppressive adhesion-molecule blocker natalizumab.
Carrat, F. & Flahault, A. Influenza vaccine: the challenge of antigenic drift. Vaccine 25, 6852–6862 (2007).
Wong, K. T. Emerging epidemic viral encephalitides with a special focus on henipaviruses. Acta Neuropathol. 120, 317–325 (2010).
Astrom, K. E., Mancall, E. L. & Richardson, E. P. Jr. Progressive multifocal leuko-encephalopathy: a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 81, 93–111 (1958).
Cinque, P., Koralnik, I. J., Gerevini, S., Miro, J. M. & Price, R. W. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect. Dis. 9, 625–636 (2009).
Focosi, D. et al. Progressive multifocal leukoencephalopathy: what's new? Neuroscientist 16, 308–323 (2010).
Sunyaev, S. R., Lugovskoy, A., Simon, K. & Gorelik, L. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML). PLoS Genet. 5, e1000368 (2009).
Tan, C. S. et al. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J. Virol. 84, 9200–9209 (2010).
Tan, C. S. & Koralnik, I. J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 9, 425–437 (2010).
Aksamit, A. J., Mourrain, P., Sever, J. L. & Major, E. O. Progressive multifocal leukoencephalopathy: investigation of three cases using in situ hybridization with JC virus biotinylated DNA probe. Ann. Neurol. 18, 490–496 (1985).
Pho, M. T., Ashok, A. & Atwood, W. J. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74, 2288–2292 (2000).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) intramural program. S.S.K. is presently supported by a NIH National Research Service Award (NS061447-01). We would like to thank J. Kim and M. Dustin at New York University for insightful discussions and a very supportive collaboration focused on imaging CNS antiviral immunity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (Movie)
CNS immune sentinels. (MOV 37922 kb)
Supplementary information S2 (Movie)
3D projections of the meninges, glial limitans and BBB. (MOV 24025 kb)
Supplementary information S3 (Movie)
Distribution of LCMV in meningeal stroma. (MOV 9580 kb)
Supplementary information S4 (Movie)
Viral meningitis in 4D. (MOV 27455 kb)
Supplementary information S5 (Movie)
Vascular occlusion and breakdown during LCMV meningitis. (MOV 8191 kb)
Glossary
- Immune-privileged
-
A term used to describe areas of the body with a decreased inflammatory response to foreign antigens, including tissue grafts. These sites include the brain, eye, testis and placenta.
- Blood–brain barrier
-
A barrier formed by tight junctions between endothelial cells that markedly limits entry to the CNS by leukocytes and all large molecules, including to some extent immunoglobulins, cytokines and complement proteins.
- Meninges
-
The membranes surrounding the brain and spinal cord. There are three layers of meninges: the dura mater (outer), the arachnoid mater (middle) and the pia mater (inner).
- Aseptic meningitis
-
Infection and inflammation of the meninges that is not caused by bacteria. Enteroviruses such as echovirus and coxsackie virus are the most common cause of viral meningitis, but cytomegalovirus, HSV, HIV, JEV, LCMV, mumps virus, rabies virus, VZV and WNV can also cause the disease.
- Encephalitis
-
Infection and inflammation of the brain parenchyma. This can be caused by adenovirus, cytomegalovirus, coxsackievirus, EBV, echovirus, HSV, measles virus, poliovirus, mumps virus, rabies virus, rubella virus, VZV and WNV.
- Meningoencephalitis
-
A disease that resembles both meningitis and encephalitis and is characterized by infection and inflammation of both the meninges and brain parenchyma.
- Two-photon laser scanning microscopy
-
(TPLSM). Laser scanning microscopy that uses pulsed infrared laser light for the excitation of conventional fluorophores or fluorescent proteins. This technique greatly reduces photodamage to living specimens and improves the depth of tissue penetration, owing to the low level of light scattering within the tissue.
- Tight junctions
-
A belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight-junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludins proteins.
- Pericytes
-
Cells embedded in the vascular basement membrane of microvessels that are thought to be derived from the vascular smooth muscle lineage. They make close cellular contact with endothelial cells and this interaction is essential for the maintenance of vessel function, as well as for the regulation of angiogenesis and vascular remodelling.
- Anterograde and retrograde transport systems
-
Cargo is moved between the cell body (soma) and the synapse of neurons using two transport mechanisms. The anterograde transport system uses kinesin motors to move cargo from the cell body to the synapse, whereas the retrograde system moves material from the synapse back to the cell body using dynein.
- Antigenic drift
-
A process by which circulating influenza viruses are constantly changing, which allows the virus to cause annual epidemics of illness. Antigenic drift occurs when mutations accumulate in the haemagglutinin and neuraminidase genes and alter the antigenicity of these proteins such that the 'drifted' strains are no longer neutralized by antibodies that were specific for previously circulating strains.
- Pathogen-associated molecular patterns
-
(PAMPs). Molecular patterns that are found in pathogens but not in mammalian cells. Examples include terminally mannosylated and polymannosylated compounds (which bind the mannose receptor) and various microbial components, such as bacterial lipopolysaccharide, hypomethylated DNA, flagellin and double-stranded RNA (all of which bind Toll-like receptors).
- γδ T cells
-
T cells that express the γδ T cell receptor. These T cells are present in the skin, vagina and intestinal epithelium as intraepithelial lymphocytes.
- MicroRNAs
-
(miRNAs). Small RNA molecules that regulate the expression of genes by binding to the 3′-untranslated regions (3′-UTRs) of specific mRNAs.
- Quantum dot
-
A nanocrystalline semiconductor of extremely small size (5–50 nm in diameter) that absorbs incident photons and then emits light of a slightly longer wavelength. Because of a phenomenon called the quantum confinement effect, the colour (wavelength) of the emitted light is determined by the size of the nanocrystal.
Rights and permissions
About this article
Cite this article
McGavern, D., Kang, S. Illuminating viral infections in the nervous system. Nat Rev Immunol 11, 318–329 (2011). https://doi.org/10.1038/nri2971
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2971
This article is cited by
-
Fusobacterium nucleatum bacteremia complicated with intracranial Porphyromonas gingivalis and HSV-1 infection: a case report and literature review
BMC Infectious Diseases (2024)
-
The neurobiology of SARS-CoV-2 infection
Nature Reviews Neuroscience (2024)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
-
Association of mTOR Pathway and Conformational Alterations in C-Reactive Protein in Neurodegenerative Diseases and Infections
Cellular and Molecular Neurobiology (2023)
-
Development of a multiplex droplet digital PCR assay for detection of enterovirus, parechovirus, herpes simplex virus 1 and 2 simultaneously for diagnosis of viral CNS infections
Virology Journal (2022)