Key Points
-
The LIM domain is a modular protein-binding interface that is found in a wide variety of eukaryotic proteins with diverse biological functions.
-
The LIM domain can be identified by a conserved sequence: C(X)2C(X)16–23(H/C)X2,4(C/H/E)(X)2C(X)2C(X)14–21(C/H)(X)2/1/3(C/H/D/E)X. Text that is not bold denotes infrequently observed patterns (which are seen in <10% of cases). The conserved residues are zinc-binding ligands that establish a double zinc-finger structure, X denotes any amino acid.
-
The LIM domain can bind a wide variety of protein targets. The coordination of zinc provides the LIM domain with a stable framework, whereas the variable portions of the LIM sequence allow for tailoring of high-affinity binding to many structurally and functionally different protein partners.
-
LIM proteins function in a diverse collection of biological processes, the unifying themes of which are the control of gene expression and cytoskeletal function.
-
The molecular function of LIM domains and LIM proteins is dependent upon the binding of target proteins. Through binding, LIM domains can function as adaptors, competitors, autoinhibitors or localizers. Often, they carry out combinations of these functions.
-
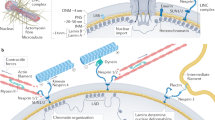
An increasing number of LIM proteins has been observed in both the nuclear and the cytoskeletal compartments. An emerging area of investigation is the potential for such proteins to communicate information between these compartments, regulating gene expression to affect cytoskeletal dynamics, and vice versa.
Abstract
First described 15 years ago as a cysteine-rich sequence that was common to a small group of homeodomain transcription factors, the LIM domain is now recognized as a tandem zinc-finger structure that functions as a modular protein-binding interface. LIM domains are present in many proteins that have diverse cellular roles as regulators of gene expression, cytoarchitecture, cell adhesion, cell motility and signal transduction. An emerging theme is that LIM proteins might function as biosensors that mediate communication between the cytosolic and the nuclear compartments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pawson, T. & Nash, P. Assembly of cell regulatory systems through protein interaction domains. Science 300, 445–452 (2003).
Way, J. C. & Chalfie, M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell 54, 5–16 (1988). The first report of a LIM sequence, which, along with references 3 and 4, provides the historical context of this sequence.
Freyd, G., Kim, S. K. & Horvitz, H. R. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature 344, 876–879 (1990).
Karlsson, O., Thor, S., Norberg, T., Ohlsson, H. & Edlund, T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature 344, 879–882 (1990).
Schmeichel, K. L. & Beckerle, M. C. The LIM domain is a modular protein-binding interface. Cell 79, 211–219 (1994). Established LIM function in protein binding.
Li, P. M., Reichert, J., Freyd, G., Horvitz, H. R. & Walsh, C. T. The LIM region of a presumptive Caenorhabditis elegans transcription factor is an iron-sulfur- and zinc-containing metallodomain. Proc. Natl Acad. Sci. USA 88, 9210–9213 (1991).
Michelsen, J. W., Schmeichel, K. L., Beckerle, M. C. & Winge, D. R. The LIM motif defines a specific zinc-binding protein domain. Proc. Natl Acad. Sci. USA 90, 4404–4408 (1993).
Michelsen, J. W. et al. Mutational analysis of the metal sites in an LIM domain. J. Biol. Chem. 269, 11108–11113 (1994).
Schmeichel, K. L. & Beckerle, M. C. Molecular dissection of a LIM domain. Mol. Biol. Cell 8, 219–230 (1997).
Hobert, O. & Westphal, H. Functions of LIM-homeobox genes. Trends Genet. 16, 75–83 (2000).
Cattaruzza, M., Lattrich, C. & Hecker, M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension 4, 726–730 (2004).
Muller, J. M. et al. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 21, 736–748 (2002). References 11 and 12 describe triggers for nucleo-cytoplasmic shuttling.
Chang, D. F. et al. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev. Cell 4, 107–118 (2003). Experiments that show the assembly of a functional complex on the CRP adaptors.
Pawson, T. Specificity in signal transduction: from phosphotyrosine–SH2 domain interactions to complex cellular systems. Cell 116, 191–203 (2004).
Perez-Alvarado, G. C. et al. Structure of the carboxy-terminal LIM domain from the cysteine rich protein CRP. Nature Struct. Biol. 1, 388–398 (1994). The first report of LIM-domain structure.
Perez-Alvarado, G. C. et al. Structure of the cysteine-rich intestinal protein, CRIP. J. Mol. Biol. 257, 153–174 (1996).
Konrat, R., Weiskirchen, R., Krautler, B. & Bister, K. Solution structure of the carboxyl-terminal LIM domain from quail cysteine-rich protein CRP2. J. Biol. Chem. 272, 12001–12007 (1997).
Kontaxis, G., Konrat, R., Krautler, B., Weiskirchen, R. & Bister, K. Structure and intramodular dynamics of the amino-terminal LIM domain from quail cysteine- and glycine-rich protein CRP2. Biochemistry 37, 7127–7134 (1998).
Velyvis, A., Yang, Y., Wu, C. & Qin, J. Solution structure of the focal adhesion adaptor PINCH LIM1 domain and characterization of its interaction with the integrin-linked kinase ankyrin repeat domain. J. Biol. Chem. 276, 4932–4939 (2001). The first report of a target-occupied LIM-domain structure describing the identification of a binding interface.
Velyvis, A. et al. Structural and functional insights into PINCH LIM4 domain-mediated integrin signaling. Nature Struct. Biol. 10, 558–564 (2003).
Deane, J. E. et al. Structural basis for the recognition of ldb1 by the N-terminal LIM domains of LMO2 and LMO4. EMBO J. 22, 2224–2233 (2003).
Deane, J. E. et al. Tandem LIM domains provide synergistic binding in the LMO4:Ldb1 complex. EMBO J. 23, 3589–3598 (2004). Describes the first crystal structure of a LIM protein.
Kosa, J. L. et al. Common metal ion coordination in LIM domain proteins. Biochemistry 33, 468–477 (1994).
Yao, X. et al. Solution structure of the chicken cysteine-rich protein, CRP1, a double-LIM protein implicated in muscle differentiation. Biochemistry 38, 5701–5713 (1999).
Konrat, R., Krautler, B., Weiskirchen, R. & Bister, K. Structure of cysteine- and glycine-rich protein CRP2. Backbone dynamics reveal motional freedom and independent spatial orientation of the LIM domains. J. Biol. Chem. 273, 23233–23240 (1998).
Arber, S. & Caroni, P. Specificity of single LIM motifs in targeting and LIM/LIM interactions in situ. Genes Dev. 10, 289–300 (1996).
Feuerstein, R., Wang, X., Song, D., Cooke, N. E. & Liebhaber, S. A. The LIM/double zinc-finger motif functions as a protein dimerization domain. Proc. Natl Acad. Sci. USA 91, 10655–10659 (1994).
Wu, R. et al. Specificity of LIM domain interactions with receptor tyrosine kinases. J. Biol. Chem. 271, 15934–15941 (1996).
Wadman, I. A. et al. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 16, 3145–3157 (1997).
Sum, E. Y. et al. The LIM domain protein LMO4 interacts with the cofactor CtIP and the tumor suppressor BRCA1 and inhibits BRCA1 activity. J. Biol. Chem. 277, 7849–7856 (2002).
Jurata, L. W. & Gill, G. N. Functional analysis of the nuclear LIM domain interactor NLI. Mol. Cell. Biol. 17, 5688–5698 (1997).
Jurata, L. W., Kenny, D. A. & Gill, G. N. Nuclear LIM interactor, a rhombotin and LIM homeodomain interacting protein, is expressed early in neuronal development. Proc. Natl Acad. Sci. USA 93, 11693–11698 (1996).
Breen, J. J., Agulnick, A. D., Westphal, H. & Dawid, I. B. Interactions between LIM domains and the LIM domain-binding protein Ldb1. J. Biol. Chem. 273, 4712–4717 (1998).
Agulnick, A. D. et al. Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature 384, 270–272 (1996).
Coleman, J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 61, 897–946 (1992).
Xue, D., Tu, Y. & Chalfie, M. Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science 261, 1324–1328 (1993).
Baltz, R., Evrard, J., Bourdon, V. & Steinmetz, A. The pollen-specific LIM protein PLIM-1 from sunflower binds nucleic acids in vitro. Sex. Plant Reprod. 9, 264–268 (1996).
Nishiya, N., Sabe, H., Nose, K. & Shibanuma, M. The LIM domains of hic-5 protein recognize specific DNA fragments in a zinc-dependent manner in vitro. Nucleic Acids Res. 26, 4267–4273 (1998).
Maul, R. S. & Chang, D. D. EPLIN, epithelial protein lost in neoplasm. Oncogene 18, 7838–7841 (1999).
Maul, R. S. et al. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J. Cell Biol. 160, 399–407 (2003).
Xia, H., Winokur, S. T., Kuo, W. L., Altherr, M. R. & Bredt, D. S. Actinin-associated LIM protein: identification of a domain interaction between PDZ and spectrin-like repeat motifs. J. Cell Biol. 139, 507–515 (1997).
Pomies, P., Macalma, T. & Beckerle, M. C. Purification and characterization of an α-actinin-binding PDZ–LIM protein that is up-regulated during muscle differentiation. J. Biol. Chem. 274, 29242–29250 (1999).
Pashmforoush, M. et al. Adult mice deficient in actinin-associated LIM-domain protein reveal a developmental pathway for right ventricular cardiomyopathy. Nature Med. 7, 591–597 (2001).
Faulkner, G. et al. ZASP: a new Z-band alternatively spliced PDZ-motif protein. J. Cell Biol. 146, 465–475 (1999).
Passier, R., Richardson, J. A. & Olson, E. N. Oracle, a novel PDZ–LIM domain protein expressed in heart and skeletal muscle. Mech. Dev. 92, 277–284 (2000).
Zhou, Q., Ruiz-Lozano, P., Martone, M. E. & Chen, J. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to α-actinin-2 and protein kinase C. J. Biol. Chem. 274, 19807–19813 (1999).
Frey, N. & Olson, E. N. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J. Biol. Chem. 277, 13998–14004 (2002).
Vatta, M. et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J. Am. Coll. Cardiol. 42, 2014–2027 (2003).
Arimura, T. et al. A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. J. Biol. Chem. 279, 6746–6752 (2004).
Zhou, Q. et al. Ablation of Cypher, a PDZ–LIM domain Z-line protein, causes a severe form of congenital myopathy. J. Cell Biol. 155, 605–612 (2001).
Suzuki, T. et al. MICAL, a novel CasL interacting molecule, associates with vimentin. J. Biol. Chem. 277, 14933–14941 (2002).
O'Neill, G. M., Fashena, S. J. & Golemis, E. A. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 10, 111–119 (2000).
Terman, J. R., Mao, T., Pasterkamp, R. J., Yu, H. H. & Kolodkin, A. L. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109, 887–900 (2002).
Lundquist, E. A., Herman, R. K., Shaw, J. E. & Bargmann, C. I. UNC-115, a conserved protein with predicted LIM and actin-binding domains, mediates axon guidance in C. elegans. Neuron 21, 385–392 (1998).
Struckhoff, E. C. & Lundquist, E. A. The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C. elegans. Development 130, 693–704 (2003).
Labouesse, M. & Georges-Labouesse, E. Cell adhesion: parallels between vertebrate and invertebrate focal adhesions. Curr. Biol. 13, R528–R530 (2003).
Tu, Y., Wu, S., Shi, X., Chen, K. & Wu, C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47 (2003).
Takafuta, T., Saeki, M., Fujimoto, T. T., Fujimura, K. & Shapiro, S. S. A new member of the LIM protein family binds to filamin B and localizes at stress fibers. J. Biol. Chem. 278, 12175–12181 (2003).
Tadokoro, S. et al. Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 (2003).
Luo, G., Herrera, A. H. & Horowits, R. Molecular interactions of N-RAP, a nebulin-related protein of striated muscle myotendon junctions and intercalated disks. Biochemistry 38, 6135–6143 (1999).
Schaller, M. D. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20, 6459–6472 (2001).
Webb, D. J. et al. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 6, 154–161 (2004).
Kasai, M. et al. The Group 3 LIM domain protein paxillin potentiates androgen receptor transactivation in prostate cancer cell lines. Cancer Res. 63, 4927–4935 (2003).
Fujimoto, N. et al. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J. Biol. Chem. 274, 8316–8321 (1999).
Yang, L., Guerrero, J., Hong, H., DeFranco, D. B. & Stallcup, M. R. Interaction of the tau2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol. Biol. Cell 11, 2007–2018 (2000).
Wu, C. Integrin-linked kinase and PINCH: partners in regulation of cell–extracellular matrix interaction and signal transduction. J. Cell Sci. 112, 4485–4489 (1999).
Tu, Y., Li, F., Goicoechea, S. & Wu, C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell. Biol. 19, 2425–2434 (1999).
Tu, Y., Li, F. & Wu, C. Nck-2, a novel Src homology 2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol. Biol. Cell 9, 3367–3382 (1998).
Clark, K. A., McGrail, M. & Beckerle, M. C. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130, 2611–2621 (2003).
Hobert, O., Moerman, D. G., Clark, K. A., Beckerle, M. C. & Ruvkun, G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J. Cell Biol. 144, 45–57 (1999).
Campana, W. M., Myers, R. R. & Rearden, A. Identification of PINCH in Schwann cells and DRG neurons: shuttling and signaling after nerve injury. Glia 41, 213–223 (2003).
Shirasaki, R. & Pfaff, S. L. Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci. 25, 251–281 (2002).
Jurata, L. W., Pfaff, S. L. & Gill, G. N. The nuclear LIM domain interactor NLI mediates homo- and heterodimerization of LIM domain transcription factors. J. Biol. Chem. 273, 3152–3157 (1998).
Thaler, J. P., Lee, S. K., Jurata, L. W., Gill, G. N. & Pfaff, S. L. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein–protein interactions. Cell 110, 237–249 (2002). Elegant use of chimeric proteins to further define the LIM combinatorial code.
Fimia, G. M., de Cesare, D. & Sassone-Corsi, P. A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol. Cell Biol. 20, 8613–8622 (2000).
Wixler, V. et al. The LIM-only protein DRAL/FHL2 binds to the cytoplasmic domain of several α and β integrin chains and is recruited to adhesion complexes. J. Biol. Chem. 275, 33669–33678 (2000).
Li, H. Y. et al. Translocation of a human focal adhesion LIM-only protein, FHL2, during myofibrillogenesis and identification of LIM2 as the principal determinants of FHL2 focal adhesion localization. Cell Motil. Cytoskeleton 48, 11–23 (2001).
Warren, A. J. et al. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell 78, 45–57 (1994).
Xu, Z., Huang, S., Chang, L. S., Agulnick, A. D. & Brandt, S. J. Identification of a TAL1 target gene reveals a positive role for the LIM domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol. Cell. Biol. 23, 7585–7599 (2003). Identifies a target gene for intact LDB1–LMO2 regulatory complexes with tandem DNA recognition sites.
Louis, H. A., Pino, J. D., Schmeichel, K. L., Pomies, P. & Beckerle, M. C. Comparison of three members of the cysteine-rich protein family reveals functional conservation and divergent patterns of gene expression. J. Biol. Chem. 272, 27484–27491 (1997).
Knoll, R. et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111, 943–955 (2002). Proposes a model for the role of MLP in nucleo-cytoplasmic shuttling.
Ecarnot-Laubriet, A. et al. Downregulation and nuclear relocation of MLP during the progression of right ventricular hypertrophy induced by chronic pressure overload. J. Mol. Cell. Cardiol. 32, 2385–2395 (2000).
Milan, M. & Cohen, S. M. Temporal regulation of apterous activity during development of the Drosophila wing. Development 127, 3069–3078 (2000).
Weihe, U., Milan, M. & Cohen, S. M. Regulation of Apterous activity in Drosophila wing development. Development 128, 4615–4622 (2001).
Milan, M. & Cohen, S. M. Regulation of LIM homeodomain activity in vivo: a tetramer of dLDB and apterous confers activity and capacity for regulation by dLMO. Mol. Cell 4, 267–273 (1999).
Milan, M., Diaz-Benjumea, F. J. & Cohen, S. M. Beadex encodes an LMO protein that regulates Apterous LIM-homeodomain activity in Drosophila wing development: a model for LMO oncogene function. Genes Dev. 12, 2912–2920 (1998).
van Meyel, D. J. et al. Chip and apterous physically interact to form a functional complex during Drosophila development. Mol. Cell 4, 259–265 (1999).
Ostendorff, H. P. et al. Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature 416, 99–103 (2002).
Hiratani, I., Yamamoto, N., Mochizuki, T., Ohmori, S. Y. & Taira, M. Selective degradation of excess Ldb1 by Rnf12/RLIM confers proper Ldb1 expression levels and Xlim-1/Ldb1 stoichiometry in Xenopus organizer functions. Development 130, 4161–4175 (2003).
Pufall, M. A. & Graves, B. J. Autoinhibitory domains: modular effectors of cellular regulation. Annu. Rev. Cell Dev. Biol. 18, 421–462 (2002).
Arber, S. et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393, 805–809 (1998).
Yang, N. et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393, 809–812 (1998). References 91 and 92 first established LIM function in actin dynamics.
Yang, N. & Mizuno, K. Nuclear export of LIM-kinase 1, mediated by two leucine-rich nuclear-export signals within the PDZ domain. Biochem. J. 338, 793–798 (1999).
Roovers, K., Klein, E. A., Castagnino, P. & Assoian, R. K. Nuclear translocation of LIM kinase mediates Rho–Rho kinase regulation of cyclin D1 expression. Dev. Cell 5, 273–284 (2003).
Nagata, K., Ohashi, K., Yang, N. & Mizuno, K. The N-terminal LIM domain negatively regulates the kinase activity of LIM-kinase 1. Biochem. J. 343, 99–105 (1999).
Ohashi, K. et al. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem. 275, 3577–3582 (2000).
Edwards, D. C., Sanders, L. C., Bokoch, G. M. & Gill, G. N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nature Cell Biol. 1, 253–259 (1999).
Tobias, E. S., Hurlstone, A. F., MacKenzie, E., McFarlane, R. & Black, D. M. The TES gene at 7q31.1 is methylated in tumours and encodes a novel growth-suppressing LIM domain protein. Oncogene 20, 2844–2853 (2001).
Gubb, D. et al. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 13, 2315–2327 (1999).
Garvalov, B. K. et al. The conformational state of Tes regulates its zyxin-dependent recruitment to focal adhesions. J. Cell Biol. 161, 33–39 (2003).
Cyert, M. S. Regulation of nuclear localization during signaling. J. Biol. Chem. 276, 20805–20808 (2001).
Nix, D. A. et al. Targeting of zyxin to sites of actin membrane interaction and to the nucleus. J. Biol. Chem. 276, 34759–34767 (2001). The first careful examination of LIM nucleo-cytoplasmic shuttling.
Drees, B. et al. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J. Biol. Chem. 275, 22503–22511 (2000).
Drees, B. E., Andrews, K. M. & Beckerle, M. C. Molecular dissection of zyxin function reveals its involvement in cell motility. J. Cell Biol. 147, 1549–1560 (1999).
Nix, D. A. & Beckerle, M. C. Nuclear–cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J. Cell Biol. 138, 1139–1147 (1997).
Petit, M. M., Meulemans, S. M. & Van de Ven, W. J. The focal adhesion and nuclear targeting capacity of the LIM-containing lipoma-preferred partner (LPP) protein. J. Biol. Chem. 278, 2157–2168 (2003).
Wang, Y. & Gilmore, T. D. LIM domain protein Trip6 has a conserved nuclear export signal, nuclear targeting sequences, and multiple transactivation domains. Biochim. Biophys. Acta 1538, 260–272 (2001).
Kanungo, J., Pratt, S. J., Marie, H. & Longmore, G. D. Ajuba, a cytosolic LIM protein, shuttles into the nucleus and affects embryonal cell proliferation and fate decisions. Mol. Biol. Cell 11, 3299–3313 (2000).
Srichai, M. B. et al. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J. Biol. Chem. 279, 14398–14408 (2004).
Akazawa, H. et al. A novel LIM protein Cal promotes cardiac differentiation by association with CSX/NKX2-5. J. Cell Biol. 164, 395–405 (2004).
Shibanuma, M. et al. Hic-5 communicates between focal adhesions and the nucleus through oxidant-sensitive nuclear export signal. Mol. Biol. Cell 14, 1158–1171 (2003).
Krishna, S. S., Majumdar, I. & Grishin, N. V. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 31, 532–550 (2003).
Grishin, N. V. Treble clef finger — a functionally diverse zinc-binding structural motif. Nucleic Acids Res. 29, 1703–1714 (2001).
Schlessinger, J. & Lemmon, M. A. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 191, RE12 (2003).
Nourry, C., Grant, S. G. & Borg, J. P. PDZ domain proteins: plug and play! Sci. STKE, RE7 (2003).
Gimona, M., Djinovic-Carugo, K., Kranewitter, W. J. & Winder, S. J. Functional plasticity of CH domains. FEBS Lett. 513, 98–106 (2002).
Vardar, D. et al. Villin-type headpiece domains show a wide range of F-actin-binding affinities. Cell Motil. Cytoskeleton 52, 9–21 (2002).
Kobe, B. & Kajava, A. V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 (2001).
Mosavi, L. K., Minor, D. L. Jr & Peng, Z. Y. Consensus-derived structural determinants of the ankyrin repeat motif. Proc. Natl Acad. Sci. USA 99, 16029–16034 (2002).
Acknowledgements
The authors would like to thank M. Ranall and P. Renfranz for critical reading of the manuscript, and H. Schubert for assistance with the illustration of the three-dimensional protein structures. We are also grateful to J. Matthews for sharing structural data in advance of publication. This work was supported by the Huntsman Cancer Foundation and National Institutes of Health grants to M.C.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- HOMEODOMAIN TRANSCRIPTION FACTORS
-
Transcription factors that are crucial for development, and that possess conserved, 60-amino-acid DNA-binding domains.
- SH2 DOMAIN
-
(Src-homology-2 domain). A protein motif that recognizes and binds tyrosine-phosphorylated sequences, and thereby has a key role in relaying cascades of signal transduction.
- SH3 DOMAIN
-
(Src-homology-3 domain). A protein sequence of 50 amino acids that recognizes and binds amino-acid sequences rich in proline.
- LD MOTIF
-
A short sequence found within proteins that has the consensus sequence LDXLLXXL and functions as a protein-binding interface.
- PDZ DOMAIN
-
A protein-interaction domain that often occurs in scaffolding proteins, and is named after the founding members of this protein family (PSD95, Discs large and ZO-1).
- RUBREDOXIN-TYPE ZINC KNUCKLES
-
A tight-turn structure within proteins formed by coordination of a metal ion by two closely spaced cysteine residues in the primary sequence. Rubredoxin is a small iron-sulphur protein whose structure was among the first to be solved by X-ray crystallography.
- GATA-TYPE TRANSCRIPTION FACTORS
-
A family of transcription factors that contain a zinc-finger motif that was first identified in the vertebrate GATA1 protein. These transcription factors bind the consensus sequence GATA in the regulatory regions of genes.
- WD MOTIF
-
A repeat of ∼40 amino acids with a characteristic central tryptophan–aspartic-acid motif that can recognize and bind protein targets containing phosphorylated threonine.
- RING DOMAIN
-
A cysteine-rich tandem zinc-finger domain of 40–60 amino acids often found in E3 ubiquitin ligases.
- PHD
-
The plant homeodomain (PHD) zinc finger is found in many nuclear proteins that are thought to be involved in chromatin-mediated transcriptional regulation.
- FYVE
-
A zinc-finger-containing protein motif that binds the membrane lipid phosphatidylinositol-3-phosphate. The protein that contains FYVE is thereby targeted to the membrane.
- NMR CHEMICAL SHIFT ANALYSIS
-
Changes in the NMR signature of a protein that are induced on addition of an NMR silent binding partner. Amino-acid residues that participate in binding have altered chemical shifts.
- Arp2/3 COMPLEX
-
A complex that consists of two actin-related proteins ARP2 and ARP3, along with five smaller proteins. When activated, the ARP2/3 complex binds to the side of an existing actin filament and nucleates the assembly of a new actin filament. The resulting branch structure is Y-shaped.
- STRESS FIBRE
-
An axial bundle of F-actin and myosin that traverses the cytoplasm. The formation of stress fibres is typically induced by the activity of the GTPase RhoA.
- MEMBRANE RUFFLES
-
Processes that are formed by the movement of lamellipodia in the dynamic process of folding back onto the cell body from which they have extended.
- CARDIOMYOPATHY
-
A disease of the heart muscle.
- Z-LINE
-
A region at the boundaries of muscle sarcomeres in which the actin filaments are anchored. It appears as a dark transverse line in electron micrographs.
- SARCOMERE
-
The basic structural and functional contractile unit of muscle, composed of actin and myosin.
- GROWTH CONE
-
Motile tip of the axon or dendrite of a growing nerve cell, which spreads out into a large cone-shaped appendage.
- MONOOXYGENASE DOMAIN
-
A protein domain that catalyses oxidoreduction reactions using a flavin cofactor.
- CALPONIN-HOMOLOGY DOMAIN
-
A protein domain, often found tandemly arrayed, that functions in the binding of actin.
- RHO FAMILY GTPases
-
Ras-related small GTPases involved in controlling the polymerization of actin.
- NEBULIN SUPER-REPEATS
-
A 35-residue motif, found in a uniform repeating pattern along the length of the sequence of nebulin. The motif has a role in binding and stabilizing F-actin.
- MYOFIBRIL
-
The structural unit of striated muscle fibres. Several myofibrils make up each fibre.
- SARCOLEMMA
-
The plasma membrane (plasmalemma) of a muscle cell.
- SCHWANN CELLS
-
Cells that produce the myelin sheath around axons in the peripheral nervous system.
- INTERNEURONS
-
Small neurons within the central nervous system that function as connectors between two neurons.
- E-BOX DNA
-
DNA in the regulatory regions of genes with the sequence CAGATG, specifically recognized by basic helix–loop–helix transcription factors.
- SERUM-RESPONSE FACTOR
-
(SRF). A MADS-domain-containing transcription factor that binds to the serum-response element in the promoter-enhancer region of many genes.
- PROTEASOME
-
A protein complex that is responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin.
- E3 UBIQUITIN PROTEIN LIGASE
-
The third enzyme in a series responsible for ubiquitylation and subsequent degradation of target proteins. E3 enzymes, which are numerous, provide platforms for binding target substrates, thereby conferring specificity to this process.
- PLANAR POLARITY
-
The pattern of organization of cells within the plane of an epithelium.
Rights and permissions
About this article
Cite this article
Kadrmas, J., Beckerle, M. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol 5, 920–931 (2004). https://doi.org/10.1038/nrm1499
Issue Date:
DOI: https://doi.org/10.1038/nrm1499
This article is cited by
-
Identification of novel FHL1 mutations associated with X-linked scapuloperoneal myopathy in unrelated Chinese patients
Journal of Human Genetics (2023)
-
Proteomic analysis of the effect of hemin in breast cancer
Scientific Reports (2023)
-
Mena regulates nesprin-2 to control actin–nuclear lamina associations, trans-nuclear membrane signalling and gene expression
Nature Communications (2023)
-
The role of prickle proteins in vertebrate development and pathology
Molecular and Cellular Biochemistry (2023)
-
Genome-Wide Identification and Characterization of LIM Gene Family in Grapevine (Vitis vinifera L.) and Their Expression Analysis at Early Bud Developmental Stages
Plant Molecular Biology Reporter (2023)