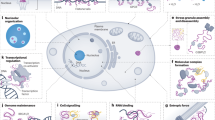
Key Points
-
Recent advances in the techniques that are used to design and prepare protein domains have led to the realization that several proteins, which are involved in important cellular processes, contain regions that are intrinsically unstructured in their normal, functional state.
-
Disordered or unstructured regions are characterized by a compositional bias in their amino-acid sequence, in that they contain a significantly larger proportion of small and hydrophilic amino acids and proline residues than structured regions.
-
Sequences with extreme compositional bias generally function as linkers between structured domains and are frequently the sites of disease-related gene truncations or translocations.
-
The presence of unstructured or incompletely folded regions in proteins that are involved in signalling, transcriptional and translational processes can be rationalized in several ways — for example, the requirement for binding with high specificity and reversibility, the requirement for binding to different partners (for example, if post-translational modification is necessary for control of function), and the requirement for rapid degradation when the signal is turned off.
-
The folding of an unstructured domain on binding to its partner can result in the formation of a complex with an extremely large surface area of interaction. This provides a specificity that could not otherwise be obtained (except by increasing the size of the protein, with the consequent increases in metabolic burden on the cell).
-
The coupled folding and binding of proteins allows a much greater range of possible interactions within the same set of proteins, and therefore provides versatility. For example, different signalling proteins can bind to a given receptor, potentiating different reactions, and a given signalling protein can bind to different receptors.
Abstract
Many gene sequences in eukaryotic genomes encode entire proteins or large segments of proteins that lack a well-structured three-dimensional fold. Disordered regions can be highly conserved between species in both composition and sequence and, contrary to the traditional view that protein function equates with a stable three-dimensional structure, disordered regions are often functional, in ways that we are only beginning to discover. Many disordered segments fold on binding to their biological targets (coupled folding and binding), whereas others constitute flexible linkers that have a role in the assembly of macromolecular arrays.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Dunker, A. K., Brown, C. J., Lawson, J. D., Iakoucheva, L. M. & Obradovic, Z. Intrinsic disorder and protein function. Biochemistry 41, 6573–6582 (2002).
Uversky, V. N. Natively unfolded proteins: a point where biology waits for physics. Protein Sci. 11, 739–756 (2002). Provides a comprehensive review of the field of unfolded proteins with much valuable analysis. It contains the most up-to-date table of unfolded proteins.
Boesch, C., Bundi, A., Oppliger, M. & Wüthrich, K. 1H nuclear-magnetic-resonance studies of the molecular conformation of monomeric glucagon in aqueous solution. Eur. J. Biochem. 91, 209–214 (1978).
Daniels, A. J., Williams, R. J. P. & Wright, P. E. The character of the stored molecules in chromaffin granules of the adrenal medulla: a nuclear magnetic resonance study. Neuroscience 3, 573–585 (1978).
Wright, P. E. & Dyson, H. J. Intrinsically unstructured proteins: re-assessing the protein structure–function paradigm. J. Mol. Biol. 293, 321–331 (1999).
Iakoucheva, L. M., Brown, C. J., Lawson, J. D., Obradovic, Z. & Dunker, A. K. Intrinsic disorder in cell-signaling and cancer-associated proteins. J. Mol. Biol. 323, 573–584 (2002).
Iakoucheva, L. M. et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 32, 1037–1049 (2004).
Tompa, P. Intrinsically unstructured proteins. Trends Biochem. Sci. 27, 527–533 (2002).
Romero, P. et al. Sequence complexity of disordered protein. Proteins 42, 38–48 (2001).
Vucetic, S., Brown, C. J., Dunker, A. K. & Obradovic, Z. Flavors of protein disorder. Proteins 52, 573–584 (2003).
Mitchell, P. J. & Tjian, R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 245, 371–378 (1989).
O'Hare, P. & Williams, G. Structural studies of the acidic transactivation domain of the Vmw65 protein of herpes simplex virus using 1H NMR. Biochemistry 31, 4150–4156 (1992).
Longhi, S. et al. The C-terminal domain of the measles virus nucleoprotein is intrinsically disordered and folds upon binding to the C-terminal moiety of the phosphoprotein. J. Biol. Chem. 278, 18638–18648 (2003).
Romero, P., Obradovic, Z., Kissinger, C. R., Villafranca, J. E. & Dunker, A. K. Identifying disordered regions in proteins from amino acid sequences. Proc. IEEE Int. Conf. Neural Netw. 1, 90–95 (1997).
Uversky, V. N., Gillespie, J. R. & Fink, A. L. Why are 'natively unfolded' proteins unstructured under physiologic conditions? Proteins 41, 415–427 (2000).
Linding, R. et al. Protein disorder prediction: implications for structural proteomics. Structure (Camb.) 11, 1453–1459 (2003).
Linding, R., Russell, R. B., Neduva, V. & Gibson, T. J. GlobPlot: exploring protein sequences for globularity and disorder. Nucleic Acids Res. 31, 3701–3708 (2003).
Ward, J. J., Sodhi, J. S., McGuffin, L. J., Buxton, B. F. & Jones, D. T. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J. Mol. Biol. 337, 635–645 (2004).
Weathers, E. A., Paulaitis, M. E., Woolf, T. B. & Hoh, J. H. Reduced amino acid alphabet is sufficient to accurately recognize intrinsically disordered protein. FEBS Lett. 576, 348–352 (2004).
Dunker, A. K. et al. Intrinsically disordered protein. J. Mol. Graph. Model. 19, 26–59 (2001).
Vucetic, S. et al. DisProt: a database of protein disorder. Bioinformatics 21, 137–140 (2005).
Tompa, P. Intrinsically unstructured proteins evolve by repeat expansion. Bioessays 25, 847–855 (2003).
Dyson, H. J. & Wright, P. E. Nuclear magnetic resonance methods for elucidation of structure and dynamics of disordered states. Methods Enzymol. 339, 258–270 (2001).
Dyson, H. J. & Wright, P. E. Unfolded proteins and protein folding studied by NMR. Chem. Rev. 104, 3607–3622 (2004).
Rose, G. D. in Advances in Protein Chemistry Vol. 62, (eds Richards, F. M., Eisenberg, D. S. & Kuriyan, J.) (Academic Press, San Diego, 2002). This book provides an excellent introduction to the biophysical techniques that can be used to identify and characterize unfolded proteins and protein domains.
Dyson, H. J. & Wright, P. E. Insights into the structure and dynamics of unfolded proteins from nuclear magnetic resonance. Adv. Protein Chem. 62, 311–340 (2002).
Rucker, A. L., Pager, C. T., Campbell, M. N., Qualls, J. E. & Creamer, T. P. Host-guest scale of left-handed polyproline II helix formation. Proteins 53, 68–75 (2003).
Shi, Z., Olson, C. A., Rose, G. D., Baldwin, R. L. & Kallenbach, N. R. Polyproline II structure in a sequence of seven alanine residues. Proc. Natl Acad. Sci. USA 99, 9190–9195 (2002).
Dunker, A. K., Obradovic, Z., Romero, P., Garner, E. C. & Brown, C. J. Intrinsic protein disorder in complete genomes. Genome Inform. Ser. Workshop Genome Inform. 11, 161–171 (2000).
Wootton, J. C. & Drummond, M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. Des. Sel. 2, 535–543 (1989).
Zhou, H. X. Quantitative account of the enhanced affinity of two linked scFvs specific for different epitopes on the same antigen. J. Mol. Biol. 329, 1–8 (2003).
Laity, J. H., Dyson, H. J. & Wright, P. E. DNA-induced α-helix capping in conserved linker sequences is a determinant of binding affinity in Cys2-His2 zinc fingers. J. Mol. Biol. 295, 719–727 (2000).
Young, M. A., Gonfloni, S., Superti-Furga, G., Roux, B. & Kuriyan, J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell 105, 115–126 (2001).
Laity, J. H., Dyson, H. J. & Wright, P. E. Molecular basis for modulation of biological function by alternate splicing of the Wilms' tumor suppressor protein. Proc. Natl Acad. Sci. USA 97, 11932–11935 (2000).
Namba, K. Roles of partly unfolded conformations in macromolecular self-assembly. Genes Cells 6, 1–12 (2001).
Tompa, P. & Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 18, 1169–1175 (2004).
Dyson, H. J. & Wright, P. E. Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 12, 54–60 (2002). Provides a comprehensive survey of protein folding and binding events.
Demchenko, A. P. Recognition between flexible protein molecules: induced and assisted folding. J. Mol. Recognit. 14, 42–61 (2001).
Radhakrishnan, I., Pérez-Alvarado, G. C., Dyson, H. J. & Wright, P. E. Conformational preferences in the Ser133-phosphorylated and non-phosphorylated forms of the kinase inducible transactivation domain of CREB. FEBS Lett. 430, 317–322 (1998).
Richards, J. P., Bächinger, H. P., Goodman, R. H. & Brennan, R. G. Analysis of the structural properties of cAMP-responsive element-binding protein (CREB) and phosphorylated CREB. J. Biol. Chem. 271, 13716–13723 (1996).
Radhakrishnan, I. et al. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91, 741–752 (1997).
Zhou, P., Lugovskoy, A. A., McCarty, J. S., Li, P. & Wagner, G. Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45. Proc. Natl Acad. Sci. USA 98, 6051–6055 (2001).
Giordano, A. & Avantaggiati, M. L. p300 and CBP: partners for life and death. J. Cell. Physiol. 181, 218–230 (1999).
Blobel, G. A. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood 95, 745–755 (2000).
Goodman, R. H. & Smolik, S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–1577 (2000).
Ponting, C. P., Blake, D. J., Davies, K. E., Kendrick-Jones, J. & Winder, S. J. ZZ and TAZ: new putative zinc fingers in dystrophin and other proteins. Trends Biochem. Sci. 21, 11–13 (1996).
De Guzman, R. N., Liu, H. Y., Martinez-Yamout, M., Dyson, H. J. & Wright, P. E. Solution structure of the TAZ2 (CH3) domain of the transcriptional adaptor protein CBP. J. Mol. Biol. 303, 243–253 (2000).
Dames, S. A., Martinez-Yamout, M., De Guzman, R. N., Dyson, H. J. & Wright, P. E. Structural basis for Hif-1α/CBP recognition in the cellular hypoxic response. Proc. Natl Acad. Sci. USA 99, 5271–5276 (2002).
Eckner, R. et al. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8, 869–884 (1994).
Avantaggiati, M. et al. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89, 1175–1184 (1997).
Lill, N. L., Grossman, S. R., Ginsberg, D., DeCaprio, J. & Livingston, D. M. Binding and modulation of p53 by p300/CBP coactivators. Nature 387, 823–827 (1997).
Gu, W., Shi, X. L. & Roeder, R. G. Synergistic activation of transcription by CBP and p53. Nature 387, 819–823 (1997).
Arany, Z. et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl Acad. Sci. USA 93, 12969–12973 (1996).
Freedman, S. J. et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1α. Proc. Natl Acad. Sci. USA 99, 5367–5372 (2002).
Gunasekaran, K., Tsai, C. J., Kumar, S., Zanuy, D. & Nussinov, R. Extended disordered proteins: targeting function with less scaffold. Trends Biochem. Sci. 28, 81–85 (2003). Points out that the extensive interfaces observed when unfolded proteins bind their targets would only be possible for fully structured proteins if they were much larger. However, increased protein size would result in greater macromolecular crowding or would require cells to be larger.
Russo, A. A., Jeffrey, P. D., Patten, A. K., Massagué, J. & Pavletich, N. P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A–Cdk2 complex. Nature 382, 325–331 (1996).
Huber, A. H. & Weis, W. I. The structure of the β-catenin/ E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 105, 391–402 (2001).
Sorenson, M. K., Ray, S. S. & Darst, S. A. Crystal structure of the flagellar σ/anti-σ complex σ28/FlgM reveals an intact σ factor in an inactive conformation. Mol. Cell 14, 127–138 (2004).
Bhattacharya, S. et al. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 13, 64–75 (1999).
Freedman, S. J. et al. Structural basis for negative regulation of hypoxia-inducible factor-1α by CITED2. Nature Struct. Biol. 10, 504–512 (2003).
De Guzman, R. N., Martinez-Yamout, M., Dyson, H. J. & Wright, P. E. Interaction of the TAZ1 domain of CREB-binding protein with the activation domain of CITED2: regulation by competition between intrinsically unstructured ligands for non-identical binding sites. J. Biol. Chem. 279, 3042–3049 (2004).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Yu, F., White, S. B., Zhao, Q. & Lee, F. S. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl Acad. Sci. USA 98, 9630–9635 (2001).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Whitelaw, M. L. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295, 858–861 (2002).
Elkins, J. M. et al. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1α. J. Biol. Chem. 278, 1802–1806 (2003).
Dann, C. E. III, Bruick, R. K. & Deisenhofer, J. Structure of factor-inhibiting hypoxia-inducible factor 1: an asparaginyl hydroxylase involved in the hypoxic response pathway. Proc. Natl Acad. Sci. USA 99, 15351–15356 (2002).
Kriwacki, R. W., Hengst, L., Tennant, L., Reed, S. I. & Wright, P. E. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc. Natl Acad. Sci. USA 93, 11504–11509 (1996).
Hon, W. et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature 417, 975–978 (2002).
Min, J. H. et al. Structure of an HIF-1α–pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889 (2002).
Chrivia, J. C. et al. Phosphorylated CREB binds specifically to nuclear protein CBP. Nature 365, 855–859 (1993).
Zor, T., Mayr, B. M., Dyson, H. J., Montminy, M. R. & Wright, P. E. Roles of phosphorylation and helix propensity in the binding of the KIX domain of CREB-binding protein by constitutive (c-Myb) and inducible (CREB) activators. J. Biol. Chem. 277, 42241–42248 (2002).
Knighton, D. R. et al. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 414–420 (1991).
Goto, N. K., Zor, T., Martinez-Yamout, M., Dyson, H. J. & Wright, P. E. Cooperativity in transcription factor binding to the coactivator CREB-binding protein (CBP). The mixed lineage leukemia protein (MLL) activation domain binds to an allosteric site on the Kix domain. J. Biol. Chem. 277, 43168–43174 (2002).
Vendel, A. C. & Lumb, K. J. NMR mapping of the HIV-1 Tat interaction surface of the KIX domain of the human coactivator CBP. Biochemistry 43, 904–908 (2004).
Campbell, K. M. & Lumb, K. J. Structurally distinct modes of recognition of the KIX domain of CBP by Jun and CREB. Biochemistry 41, 13956–13964 (2002).
Vendel, A. C., McBryant, S. J. & Lumb, K. J. KIX-mediated assembly of the CBP–CREB–HTLV-1 tax coactivator-activator complex. Biochemistry 42, 12481–12487 (2003).
Xu, W. et al. A transcriptional switch mediated by cofactor methylation. Science 294, 2507–2511 (2001).
Radhakrishnan, I. et al. Structural analyses of CREB–CBP transcriptional activator–coactivator complexes by NMR spectroscopy: implications for mapping the boundaries of structural domains. J. Mol. Biol. 287, 859–865 (1999).
Wei, Y., Horng, J. C., Vendel, A. C., Raleigh, D. P. & Lumb, K. J. Contribution to stability and folding of a buried polar residue at the CARM1 methylation site of the KIX domain of CBP. Biochemistry 42, 7044–7049 (2003).
Shapiro, L. β-catenin and its multiple partners: promiscuity explained. Nature Struct. Biol. 8, 484–487 (2001).
Daniels, D. L., Eklof, S. K. & Weis, W. I. β-catenin: molecular plasticity and drug design. Trends Biochem. Sci. 26, 672–678 (2001).
Huber, A. H., Stewart, D. B., Laurents, D. V., Nelson, W. J. & Weis, W. I. The cadherin cytoplasmic domain is unstructured in the absence of β-catenin. A possible-mechanism for regulating cadherin turnover. J. Biol. Chem. 276, 12301–12309 (2001).
Graham, T. A., Weaver, C., Mao, F., Kimelman, D. & Xu, W. Crystal structure of a β-catenin/Tcf complex. Cell 103, 885–896 (2000).
Graham, T. A., Ferkey, D. M., Mao, F., Kimelman, D. & Xu, W. Tcf4 can specifically recognize β-catenin using alternative conformations. Nature Struct. Biol. 8, 1048–1052 (2001).
Eklof Spink, K., Fridman, S. G. & Weis, W. I. Molecular mechanisms of β-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC–β-catenin complex. EMBO J. 20, 6203–6212 (2001).
Graham, T. A., Clements, W. K., Kimelman, D. & Xu, W. The crystal structure of the β-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Mol. Cell 10, 563–571 (2002).
Daniels, D. L. & Weis, W. I. ICAT inhibits β-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol. Cell 10, 573–584 (2002).
Lin, C. H. et al. A small domain of cbp/p300 binds diverse proteins. Solution structure and functional studies. Mol. Cell 8, 581–590 (2001).
Demarest, S. J., Deechongkit, S., Dyson, H. J., Evans, R. M. & Wright, P. E. Packing, specificity, and mutability at the binding interface between the p160 coactivator and CREB-binding protein. Protein Sci. 13, 203–210 (2004).
Demarest, S. J. et al. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature 415, 549–553 (2002). Presents the first example of two domains that are largely, if not completely, unfolded when free in solution, but that fold together when they interact to produce a complex of exceptionally high specificity and with a large surface area of binding.
Grossman, S. R. p300/CBP/p53 interaction and regulation of the p53 response. Eur. J. Biochem. 268, 2773–2778 (2001).
Bell, S., Klein, C., Muller, L., Hansen, S. & Buchner, J. p53 contains large unstructured regions in its native state. J. Mol. Biol. 322, 917–927 (2002).
Ayed, A. et al. Latent and active p53 are identical in conformation. Nature Struct. Biol. 8, 756–760 (2001). Provides important information on the N- and C-terminal domains of p53, which are unstructured in the context of the full-length protein, as well as isolated in solution.
Dawson, R. et al. The N-terminal domain of p53 is natively unfolded. J. Mol. Biol. 332, 1131–1141 (2003).
Prives, C. & Hall, P. A. The p53 pathway. J. Pathol. 187, 112–126 (1999).
Alarcon-Vargas, D. & Ronai, Z. p53–Mdm2 — the affair that never ends. Carcinogenesis 23, 541–547 (2002).
Mujtaba, S. et al. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol. Cell 13, 251–263 (2004).
Kamei, Y. et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85, 403–414 (1996).
Heery, D. M., Kalkhoven, E., Hoare, S. & Parker, M. G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387, 733–736 (1997).
Darimont, B. D. et al. Structure and specificity of nuclear receptor–coactivator interactions. Genes Dev. 12, 3343–3356 (1998).
Snowden, A. W., Anderson, L. A., Webster, G. A. & Perkins, N. D. A novel transcriptional repression domain mediates p21WAF1/CIP1 induction of p300 transactivation. Mol. Cell. Biol. 20, 2676–2686 (2000).
Girdwood, D. et al. p300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11, 1043–1054 (2003).
Thompson, P. R. et al. Regulation of the p300 HAT domain via a novel activation loop. Nature Struct. Mol. Biol. 11, 308–315 (2004).
Gerber, H. P. et al. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science 263, 808–811 (1994).
Shoemaker, B. A., Portman, J. J. & Wolynes, P. G. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc. Natl Acad. Sci. USA 97, 8868–8873 (2000). Describes a possible rationale for the presence of unstructured linker regions in multidomain proteins: a conformational ensemble for part of an interaction domain ensures that the volume of the surrounding solution is sampled extensively, which increases the likelihood of encountering the binding partner.
Smith, J. L. et al. Kinetic profiles of p300 occupancy in vivo predict common features of promoter structure and coactivator recruitment. Proc. Natl Acad. Sci. USA 101, 11554–11559 (2004).
Spolar, R. S. & Record, M. T. Coupling of local folding to site-specific binding of proteins to DNA. Science 263, 777–784 (1994).
Patel, L., Abate, C. & Curran, T. Altered protein conformation on DNA binding by Fos and Jun. Nature 347, 572–574 (1990).
DiNitto, J. P. & Huber, P. W. Mutual induced fit binding of Xenopus ribosomal protein L5 to 5S rRNA. J. Mol. Biol. 330, 979–992 (2003).
Salghetti, S. E., Caudy, A. A., Chenoweth, J. G. & Tansey, W. P. Regulation of transcriptional activation domain function by ubiquitin. Science 293, 1651–1653 (2001).
Venkatraman, P., Wetzel, R., Tanaka, M., Nukina, N. & Goldberg, A. L. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol. Cell 14, 95–104 (2004).
Yang, X. J. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 32, 959–976 (2004).
McCampbell, A. & Fischbeck, K. H. Polyglutamine and CBP: fatal attraction? Nature Med. 7, 528–530 (2001).
Nucifora, F. C. et al. Interference by Huntingtin and atrophin-1 with CBP-mediated transcription leading to cellular toxicity. Science 291, 2423–2428 (2001).
Karlin, S., Brocchieri, L., Bergman, A., Mrazek, J. & Gentles, A. J. Amino acid runs in eukaryotic proteomes and disease associations. Proc. Natl Acad. Sci. USA 99, 333–338 (2002).
Rost, B. & Liu, J. The PredictProtein server. Nucleic Acids Res. 31, 3300–3304 (2003).
Jenuwein, T. & Allis, C. D. Translating the histone code. Science 293, 1074–1080 (2001).
Berger, S. L. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12, 142–148 (2002).
Hartman, P. G., Chapman, G. E., Moss, T. & Bradbury, E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur. J. Biochem. 77, 45–51 (1977).
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997).
Brüschweiler, R., Liao, X. & Wright, P. E. Long-range motional restrictions in a multidomain zinc-finger protein from anisotropic tumbling. Science 268, 886–889 (1995).
Gross, J. D. et al. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 115, 739–750 (2003).
Wuttke, D. S., Foster, M. P., Case, D. A., Gottesfeld, J. M. & Wright, P. E. Solution structure of the first three zinc fingers of TFIIIA bound to the cognate DNA sequence: determinants of affinity and sequence specificity. J. Mol. Biol. 273, 183–206 (1997).
Koradi, R., Billeter, M. & Wüthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graphics 14, 51–55 (1996).
Lee, C., Kim, S. J., Jeong, D. G., Lee, S. M. & Ryu, S. E. Structure of human FIH-1 reveals a unique active site pocket and interaction sites for HIF-1 and von Hippel–Lindau. J. Biol. Chem. 278, 7558–7563 (2003).
Acknowledgements
We would like to thank past and present members of our laboratories for their contributions to the ideas that are expressed in this review. We are particularly grateful to M. Martinez-Yamout for continuing important contributions and for critically reading the manuscript. Our work is supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Interpro
Protein Data Bank
Swiss-Prot
FURTHER INFORMATION
DisEMBL — Intrinsic Protein Disorder Prediction 1.4
DISPHOS 1.3 — Disorder-Enhanced Phosphorylation Sites Predictor
DISPROT — Database of Protein Disorder
GLOBPROT 2 — Intrinsic Protein Disorder, Domain & Globularity Prediction
PONDR (Predictors of Natural Disordered Regions)
Glossary
- NMR
-
Nuclear magnetic resonance (NMR) spectroscopy provides information on the three-dimensional structure and dynamics of biological molecules in solution.
- CIRCULAR DICHROISM
-
The ultraviolet circular-dichroism spectrum uses the chirality or 'handedness' of biological molecules to provide information on secondary structure in solution.
- FLUORESCENCE SPECTROSCOPY
-
Fluorescence spectroscopy of proteins gives information on the environment of aromatic rings, and can be used in conjunction with external probes to determine the distances between atoms in a molecule.
- VIBRATIONAL CD SPECTROSCOPY
-
Vibrational circular dichroism (CD) is the chiroptical version of infra-red spectroscopy, and it gives information on the vibrations of individual bonds in a molecule.
- RAMAN SPECTROSCOPY
-
Raman spectroscopy provides information on bond vibrations that is complementary to that provided by infra-red spectroscopy.
- MOLTEN GLOBULE
-
The molten globule was originally defined with reference to the folding pathway of an ordered protein as a compact state of a protein, with native-like secondary structure but disordered tertiary structure.
- PROTEIN TRINITY
-
In terms of their structure, proteins can be defined as being in one of three states — unfolded, molten globule or folded.
- RANDOM COIL
-
This term refers to a 'statistical coil' with a random distribution of dihedral angles. In practice, no protein is ever a completely random coil, but the term is a convenient shorthand for the ensemble of conformations that occur for an unfolded protein.
- PROTEIN QUARTET
-
A similar division for protein structure as the protein trinity, but the quartet includes a pre-molten globule state as well as the unfolded, molten-globule and folded states.
- RAMACHANDRAN PLOT
-
A plot of the backbone dihedral angles φ and ψ for a polypeptide chain. Areas of low energy (greater probability) encompass angles that are observed in α-helical and β-sheet structures, and a part of the broad β-minimum is defined as the 'polyproline II' region.
- CYS2HIS2 ZINC-FINGER PROTEIN
-
The Cys2His2 zinc finger is a common structural motif. It is a small sequence motif that contains two Cys and two His residues, which coordinate a single zinc ion. Tandem repeats of zinc fingers are common.
- SH2 DOMAIN
-
The Src-homology-2 (SH2) domain, which is a peptide-binding domain of Src protein kinases, is a common structural motif. It binds peptides and proteins that contain phosphorylated Tyr residues.
- SH3 DOMAIN
-
The Src-homology-3 domain, which is a peptide-binding domain of Src protein kinases, is a common structural motif. It binds polyproline sequences.
- PARALOGUES
-
Sequences, or genes, that have originated from a common ancestral sequence, or gene, by a duplication event.
- CADHERIN
-
A Ca2+-binding membrane protein that mediates homophilic cell adhesion.
- E3 UBIQUITIN LIGASE
-
E3 (enzyme-3) ubiquitin ligases are the enzymes that are responsible for attaching ubiquitin to proteins, which can target them for destruction by the 26S proteasome.
- SIGMOIDAL UNFOLDING CURVE
-
A folded protein with a stable three-dimensional structure unfolds cooperatively on addition of denaturant — that is, all of the molecules in the ensemble change from being fully folded to fully unfolded within a small range of denaturant concentration. This produces the sigmoidal unfolding curve that is typical for a folded protein.
- HEAT-CAPACITY CHANGE
-
During thermal denaturation, a folded protein shows a typical bell-shaped transition in the plot of heat capacity versus temperature (measured, for example, by differential scanning calorimetry). Unfolded proteins do not show this behaviour.
- SUMO
-
(small ubiquitin-like modifier). SUMO proteins are ubiquitin-like proteins that post-translationally modify proteins to control their localization and activity.
- UBIQUITIN–PROTEASOME SYSTEM
-
Refers to the targeting of proteins for destruction by the 26S proteasome through the attachment of ubiquitin.
- PEST-SEQUENCE MOTIF
-
This term refers to an amino-acid sequence that is enriched in Pro (P), Glu (E), Ser (S) and Thr (T) residues. PEST domains are frequently found in signalling, regulatory and adhesion molecules.
- POLYGLUTAMINE-REPEAT DISORDER
-
This term refers to the group of, frequently genetic, diseases that arise due to the expansion of regions of repeated glutamine sequences — for example, Huntington's disease.
Rights and permissions
About this article
Cite this article
Dyson, H., Wright, P. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6, 197–208 (2005). https://doi.org/10.1038/nrm1589
Issue Date:
DOI: https://doi.org/10.1038/nrm1589
This article is cited by
-
SCAF1 drives the compositional diversity of mammalian respirasomes
Nature Structural & Molecular Biology (2024)
-
Pathogenic mutations of human phosphorylation sites affect protein–protein interactions
Nature Communications (2024)
-
Protein intrinsically disordered region prediction by combining neural architecture search and multi-objective genetic algorithm
BMC Biology (2023)
-
Intrinsic disorder in the open reading frame 2 of hepatitis E virus: a protein with multiple functions beyond viral capsid
Journal of Genetic Engineering and Biotechnology (2023)
-
Visualizing single-molecule conformational transition and binding dynamics of intrinsically disordered proteins
Nature Communications (2023)