Key Points
-
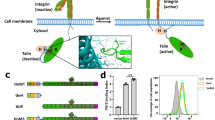
Adhesion of cells to the extracellular matrix (ECM) regulates processes that are related to cell survival, growth, differentiation, migration, polarity and proliferation. The main cell-surface receptors for ECM proteins are the integrins.
-
Integrin-linked kinase (ILK), PINCH and parvin form a ternary complex (the IPP complex) that binds to ECM-ligated integrins. This complex regulates signalling pathways and connects the ECM with the actin cytoskeleton.
-
Numerous isoforms of PINCH and parvin coexist to allow for the formation of different IPP complexes. Differential binding partners for PINCH and parvin isoforms might impart distinct functions to each complex.
-
ILK has been identified as a Ser/Thr protein kinase in vitro. In vivo confirmation of kinase activity has proven difficult because mutations in ILK disrupt the assembly and function of the IPP complex.
-
Genetic deletion of ILK, PINCH and parvin isoforms reveals common functions that are related to cell adhesion, but subtle differences in knockout phenotypes indicate separable functions outside of focal adhesions.
Abstract
The ternary complex of integrin-linked kinase (ILK), PINCH and parvin functions as a signalling platform for integrins by interfacing with the actin cytoskeleton and many diverse signalling pathways. All these proteins have synergistic functions at focal adhesions, but recent work has indicated that these proteins might also have separate roles within a cell. They function as regulators of gene transcription or cell–cell adhesion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Brown, N. H. Null mutations in the αPS2 and βPS integrin subunit genes have distinct phenotypes. Development 120, 1221–1231 (1994).
Gettner, S. N., Kenyon, C. & Reichardt, L. F. Characterization of βpat-3 heterodimers, a family of essential integrin receptors in C. elegans. J. Cell Biol. 129, 1127–1141 (1995).
Fässler, R. & Meyer, M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 9, 1896–1908 (1995).
Stephens, L. E. et al. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9, 1883–1895 (1995).
Marsden, M. & DeSimone, D. W. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development 128, 3635–3647 (2001).
Hannigan, G. E. et al. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature 379, 91–96 (1996). Identifies ILK as a novel kinase that binds to the cytoplasmic tails of β1 integrins.
Delcommenne, M. et al. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl Acad. Sci. USA 95, 11211–11216 (1998).
Persad, S. et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl Acad. Sci. USA 97, 3207–3212 (2000).
Li, F., Zhang, Y. & Wu, C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell–cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J. Cell Sci. 112, 4589–4599 (1999).
Pasquet, J. M., Noury, M. & Nurden, A. T. Evidence that the platelet integrin αIIb β3 is regulated by the integrin-linked kinase, ILK, in a PI3-kinase dependent pathway. Thromb. Haemost. 88, 115–122 (2002).
Yamaji, S. et al. Possible role of ILK–affixin complex in integrin–cytoskeleton linkage during platelet aggregation. Biochem. Biophys. Res. Commun. 297, 1324–1331 (2002).
Tu, Y., Huang, Y., Zhang, Y., Hua, Y. & Wu, C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol. 153, 585–598 (2001). References 13, 14 and 20 identify the parvins as members of the IPP complex.
Yamaji, S. et al. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell–substrate interaction. J. Cell Biol. 153, 1251–1264 (2001).
Rearden, A. A new LIM protein containing an autoepitope homologous to 'senescent cell antigen'. Biochem. Biophys. Res. Commun. 201, 1124–1131 (1994).
Tu, Y., Li, F., Goicoechea, S. & Wu, C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell Biol. 19, 2425–2434 (1999). Describes the physical interaction between ILK and PINCH1.
Hobert, O., Moerman, D. G., Clark, K. A., Beckerle, M. C. & Ruvkun, G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J. Cell Biol. 144, 45–57 (1999). Describes the UNC-97/PINCH loss-of-function phenotype in C. elegans.
Zhang, Y., Chen, K., Guo, L. & Wu, C. Characterization of PINCH-2, a new focal adhesion protein that regulates the PINCH-1–ILK interaction, cell spreading, and migration. J. Biol. Chem. 277, 38328–38338 (2002).
Braun, A. et al. PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp. Cell Res. 284, 239–250 (2003).
Nikolopoulos, S. N. & Turner, C. E. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J. Cell Biol. 151, 1435–1448 (2000).
Olski, T. M., Noegel, A. A. & Korenbaum, E. Parvin, a 42 kDa focal adhesion protein, related to the α-actinin superfamily. J. Cell Sci. 114, 525–538 (2001).
Attwell, S., Mills, J., Troussard, A., Wu, C. & Dedhar, S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol. Biol. Cell 14, 4813–4825 (2003).
Yang, Y. et al. Formation and phosphorylation of the PINCH-1–integrin linked kinase–α-parvin complex are important for regulation of renal glomerular podocyte adhesion, architecture, and survival. J. Am. Soc. Nephrol. 16, 1966–1976 (2005).
Curtis, M., Nikolopoulos, S. N. & Turner, C. E. Actopaxin is phosphorylated during mitosis and is a substrate for cyclin B1/cdc2 kinase. Biochem. J. 363, 233–242 (2002).
Clarke, D. M., Brown, M. C., LaLonde, D. P. & Turner, C. E. Phosphorylation of actopaxin regulates cell spreading and migration. J. Cell Biol. 166, 901–912 (2004).
Korenbaum, E., Olski, T. M. & Noegel, A. A. Genomic organization and expression profile of the parvin family of focal adhesion proteins in mice and humans. Gene 279, 69–79 (2001).
Zhang, Y., Chen, K., Tu, Y. & Wu, C. Distinct roles of two structurally closely related focal adhesion proteins, α-parvins and β-parvins, in regulation of cell morphology and survival. J. Biol. Chem. 279, 41695–41705 (2004). Demonstrates that cells might assemble different IPP complexes with different functions.
Zhang, Y. et al. Assembly of the PINCH–ILK–CH-ILKBP complex precedes and is essential for localization of each component to cell–matrix adhesion sites. J. Cell Sci. 115, 4777–4786 (2002).
Fukuda, T., Chen, K., Shi, X. & Wu, C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J. Biol. Chem. 278, 51324–51333 (2003). Demonstrates that the stability of the members of the IPP complex is partially dependent on the presence of the other members of the complex.
Xu, Z. et al. Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J. Biol. Chem. 280, 27631–27637 (2005).
Stanchi, F. et al. Consequences of loss of PINCH2 expression in mice. J. Cell Sci. 118, 6119–6128 (2005).
Zhang, Y., Guo, L., Chen, K. & Wu, C. A critical role of the PINCH integrin-linked kinase interaction in the regulation of cell shape change and migration. J. Biol. Chem. 277, 318–326 (2002).
Nikolopoulos, S. N. & Turner, C. E. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J. Biol. Chem. 276, 23499–23505 (2001).
Mackinnon, A. C., Qadota, H., Norman, K. R., Moerman, D. G. & Williams, B. D. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12, 787–797 (2002). Identifies the ILK orthologue in C. elegans and reveals that ILK functions as an adaptor protein in this organism.
Clark, K. A., McGrail, M. & Beckerle, M. C. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130, 2611–2621 (2003). Describes the PINCH loss-of-function phenotype in D. melanogaster.
Wu, C. PINCH, N(i)ck and the ILK: network wiring at cell–matrix adhesions. Trends Cell Biol. 15, 460–466 (2005).
Turner, C. E., Glenney, J. R. Jr. & Burridge, K. Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol. 111, 1059–1068 (1990).
Wood, C. K., Turner, C. E., Jackson, P. & Critchley, D. R. Characterisation of the paxillin-binding site and the C-terminal focal adhesion targeting sequence in vinculin. J. Cell Sci. 107, 709–717 (1994).
Kloeker, S. et al. The Kindler syndrome protein is regulated by transforming growth factor-β and involved in integrin-mediated adhesion. J. Biol. Chem. 279, 6824–6833 (2004).
Siegel, D. H. et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin–extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am. J. Hum. Genet. 73, 174–187 (2003).
Tu, Y., Wu, S., Shi, X., Chen, K. & Wu, C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47 (2003).
Feng, Y. & Walsh, C. A. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nature Cell Biol. 6, 1034–1038 (2004).
Tsuda, T., Marinetti, M. R., Masuelli, L. & Cutler, M. L. The Ras suppressor RSU-1 localizes to 10p13 and its expression in the U251 glioblastoma cell line correlates with a decrease in growth rate and tumorigenic potential. Oncogene 11, 397–403 (1995).
Vasaturo, F., Dougherty, G. W. & Cutler, M. L. Ectopic expression of Rsu-1 results in elevation of p21CIP and inhibits anchorage-independent growth of MCF7 breast cancer cells. Breast Cancer Res. Treat. 61, 69–78 (2000).
Kadrmas, J. L. et al. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J. Cell Biol. 167, 1019–1024 (2004). By combining a genetic and a biochemical approach, these authors provide a connection between PINCH and the JNK signalling pathway in D. melanogaster.
Dougherty, G. W., Chopp, T., Qi, S. M. & Cutler, M. L. The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp. Cell Res. 306, 168–179 (2005).
Bock-Marquette, I., Saxena, A., White, M. D., Dimaio, J. M. & Srivastava, D. Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432, 466–472 (2004).
Liang, X. et al. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol. Cell Biol. 25, 3056–3062 (2005). References 48 and 63 describe the Pinch1 mouse-knockout phenotype.
Velyvis, A. et al. Structural and functional insights into PINCH LIM4 domain-mediated integrin signaling. Nature Struct. Biol. 10, 558–564 (2003).
Vaynberg, J. et al. Structure of an ultraweak protein–protein complex and its crucial role in regulation of cell morphology and motility. Mol. Cell 17, 513–523 (2005).
Bladt, F. et al. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol. Cell Biol. 23, 4586–4597 (2003).
Tu, Y., Li, F. & Wu, C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol. Biol. Cell 9, 3367–3382 (1998).
Kim-Kaneyama, J., Shibanuma, M. & Nose, K. Transcriptional activation of the c-fos gene by a LIM protein, Hic-5. Biochem. Biophys. Res. Commun. 299, 360–365 (2002).
Shibanuma, M., Kim-Kaneyama, J. R., Sato, S. & Nose, K. A LIM protein, Hic-5, functions as a potential coactivator for Sp1. J. Cell. Biochem. 91, 633–645 (2004).
LaLonde, D. P., Brown, M. C., Bouverat, B. P. & Turner, C. E. Actopaxin interacts with TESK1 to regulate cell spreading on fibronectin. J. Biol. Chem. 280, 21680–21688 (2005).
Yamaji, S. et al. Affixin interacts with α-actinin and mediates integrin signaling for reorganization of F-actin induced by initial cell–substrate interaction. J. Cell Biol. 165, 539–551 (2004).
Rosenberger, G., Jantke, I., Gal, A. & Kutsche, K. Interaction of αPIX (ARHGEF6) with β-parvin (PARVB) suggests an involvement of αPIX in integrin-mediated signaling. Hum. Mol. Genet. 12, 155–167 (2003).
Manser, E. et al. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1, 183–192 (1998).
Edwards, D. C., Sanders, L. C., Bokoch, G. M. & Gill, G. N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nature Cell Biol. 1, 253–259 (1999).
Rosenberger, G., Gal, A. & Kutsche, K. αPIX associates with calpain 4, the small subunit of calpain, and has a dual role in integrin-mediated cell spreading. J. Biol. Chem. 280, 6879–6889 (2005).
Franco, S. J. et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature Cell Biol. 6, 977–983 (2004).
Matsuda, C. et al. Dysferlin interacts with affixin (β-parvin) at the sarcolemma. J. Neuropathol. Exp. Neurol. 64, 334–340 (2005).
Li, S. et al. PINCH1 regulates cell–matrix and cell–cell adhesions, cell polarity and cell survival during the peri-implantation stage. J. Cell Sci. 118, 2913–2921 (2005). Shows that PINCH1 has functional roles (cell–cell adhesion and survival) in the primitive endoderm that are independent of ILK. This indicates that ILK and PINCH1 might not always act in concert.
Campana, W. M., Myers, R. R. & Rearden, A. Identification of PINCH in Schwann cells and DRG neurons: shuttling and signaling after nerve injury. Glia 41, 213–223 (2003).
Vespa, A., Darmon, A. J., Turner, C. E., D'Souza, S. J. & Dagnino, L. Ca2+-dependent localization of integrin-linked kinase to cell junctions in differentiating keratinocytes. J. Biol. Chem. 278, 11528–11535 (2003).
Vespa, A., D'Souza, S. J. & Dagnino, L. A novel role for integrin-linked kinase in epithelial sheet morphogenesis. Mol. Biol. Cell 16, 4084–4095 (2005).
Hanks, S. K., Quinn, A. M. & Hunter, T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241, 42–52 (1988).
Hanks, S. K. & Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9, 576–596 (1995).
Lynch, D. K., Ellis, C. A., Edwards, P. A. & Hiles, I. D. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene 18, 8024–8032 (1999).
Deng, J. T., Van Lierop, J. E., Sutherland, C. & Walsh, M. P. Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J. Biol. Chem. 276, 16365–16373 (2001).
Persad, S. et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem. 276, 27462–27469 (2001). Shows that ILK phosphorylates AKT/PKB on Ser473 and reveals ILK as a candidate for HMK activity.
Leung-Hagesteijn, C., Mahendra, A., Naruszewicz, I. & Hannigan, G. E. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 20, 2160–2170 (2001).
Kumar, A. S., Naruszewicz, I., Wang, P., Leung-Hagesteijn, C. & Hannigan, G. E. ILKAP regulates ILK signaling and inhibits anchorage-independent growth. Oncogene 23, 3454–3461 (2004).
Sakai, T. et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 17, 926–940 (2003). The first report of ILK genetic deletion in the mouse. Deletion of Ilk results in severe developmental defects, but alterations in potential kinase substrates were not found.
Grashoff, C., Aszodi, A., Sakai, T., Hunziker, E. B. & Fassler, R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 4, 432–438 (2003).
Troussard, A. A. et al. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J. Biol. Chem. 278, 22374–22378 (2003).
Mills, J. et al. Role of integrin-linked kinase in nerve growth factor-stimulated neurite outgrowth. J. Neurosci. 23, 1638–1648 (2003).
Novak, A. et al. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl Acad. Sci. USA 95, 4374–4379 (1998).
Nikolopoulos, S. N. & Turner, C. E. Molecular dissection of actopaxin–integrin-linked kinase–paxillin interactions and their role in subcellular localization. J. Biol. Chem. 277, 1568–1575 (2002).
Khyrul, W. A., LaLonde, D. P., Brown, M. C., Levinson, H. & Turner, C. E. The integrin-linked kinase regulates cell morphology and motility in a rho-associated kinase-dependent manner. J. Biol. Chem. 279, 54131–54139 (2004).
Filipenko, N. R., Attwell, S., Roskelley, C. & Dedhar, S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via α-PIX. Oncogene 24, 5837–5849 (2005).
Carrera, A. C., Alexandrov, K. & Roberts, T. M. The conserved lysine of the catalytic domain of protein kinases is actively involved in the phosphotransfer reaction and not required for anchoring ATP. Proc. Natl Acad. Sci. USA 90, 442–446 (1993).
Zervas, C. G., Gregory, S. L. & Brown, N. H. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007–1018 (2001). Using an ILK loss-of-function mutation, these authors demonstrate that ILK functions as an adaptor molecule in D. melanogaster.
Fukuda, T. et al. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J. Cell Biol. 160, 1001–1008 (2003).
Tan, C. et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell 5, 79–90 (2004).
Nho, S. et al. Role of integrin-linked kinase in regulating phosphorylation of Akt and fibroblast survival in type I collagen matrices through a β1 integrin viability signaling pathway. J. Biol. Chem. 280, 26630–26639 (2005).
Lin, X., Qadota, H., Moerman, D. G. & Williams, B. D. C. elegans PAT-6/actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr. Biol. 13, 922–932 (2003). Describes the phenotypes associated with loss-of-function mutations in pat-6/parvin in C. elegans.
Rogalski, T. M., Mullen, G. P., Gilbert, M. M., Williams, B. D. & Moerman, D. G. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell–matrix adhesion structures required for integrin localization in the muscle cell membrane. J. Cell Biol. 150, 253–264 (2000).
Mercer, K. B. et al. Caenorhabditis elegans UNC-98, a C2H2 Zn finger protein, is a novel partner of UNC-97/PINCH in muscle adhesion complexes. Mol. Biol. Cell 14, 2492–2507 (2003).
Xia, Y. & Karin, M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 14, 94–101 (2004).
Ruan, W., Pang, P. & Rao, Y. The SH2/SH3 adaptor protein dock interacts with the Ste20-like kinase misshapen in controlling growth cone motility. Neuron 24, 595–605 (1999).
Peifer, M., Rauskolb, C., Williams, M., Riggleman, B. & Wieschaus, E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development 111, 1029–1043 (1991).
Staveley, B. E. et al. Genetic analysis of protein kinase B (AKT) in Drosophila. Curr. Biol. 8, 599–602 (1998).
Chun, S. J., Rasband, M. N., Sidman, R. L., Habib, A. A. & Vartanian, T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J. Cell Biol. 163, 397–408 (2003).
Friedrich, E. B. et al. Role of integrin-linked kinase in leukocyte recruitment. J. Biol. Chem. 277, 16371–16375 (2002).
Aszodi, A., Hunziker, E. B., Brakebusch, C. & Fassler, R. β1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465–2479 (2003).
Terpstra, L. et al. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J. Cell Biol. 162, 139–148 (2003).
Friedrich, E. B. et al. Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol. Cell Biol. 24, 8134–8144 (2004).
Gary, D. S., Milhavet, O., Camandola, S. & Mattson, M. P. Essential role for integrin linked kinase in Akt-mediated integrin survival signaling in hippocampal neurons. J. Neurochem. 84, 878–890 (2003).
Ishii, T., Furuoka, H., Muroi, Y. & Nishimura, M. Inactivation of integrin-linked kinase induces aberrant tau phosphorylation via sustained activation of glycogen synthase kinase 3β in N1E-115 neuroblastoma cells. J. Biol. Chem. 278, 26970–26975 (2003).
Aumailley, M., Pesch, M., Tunggal, L., Gaill, F. & Fassler, R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in β1 integrin-deficient embryoid bodies. J. Cell Sci. 113, 259–268 (2000).
Li, S. et al. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157, 1279–1290 (2002).
Wiesner, S., Legate, K. R. & Fassler, R. Integrin–actin interactions. Cell Mol. Life Sci. 62, 1081–1099 (2005).
Yasunaga, T. et al. Xenopus ILK (integrin-linked kinase) is required for morphogenetic movements during gastrulation. Genes Cells 10, 369–379 (2005).
D'Amico, M. et al. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3b and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 275, 32649–32657 (2000).
Di-Poi, N., Tan, N. S., Michalik, L., Wahli, W. & Desvergne, B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell 10, 721–733 (2002).
Di-Poi, N., Michalik, L., Tan, N. S., Desvergne, B. & Wahli, W. The anti-apoptotic role of PPARβ contributes to efficient skin wound healing. J. Steroid Biochem. Mol. Biol. 85, 257–265 (2003).
Persad, S. & Dedhar, S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 22, 375–384 (2003).
Hannigan, G., Troussard, A. A. & Dedhar, S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nature Rev. Cancer 5, 51–63 (2005).
Xu, Z., Ma, D. Z., Wang, L. Y., Su, J. M. & Zha, X. L. Transforming growth factor-β1 stimulated protein kinase B serine-473 and focal adhesion kinase tyrosine phosphorylation dependent on cell adhesion in human hepatocellular carcinoma SMMC-7721 cells. Biochem. Biophys. Res. Commun. 312, 388–396 (2003).
White, D. E., Cardiff, R. D., Dedhar, S. & Muller, W. J. Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene 20, 7064–7072 (2001).
Feng, J., Park, J., Cron, P., Hess, D. & Hemmings, B. A. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 (2004).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307, 1098–1101 (2005).
Hresko, R. C. & Mueckler, M. mTOR/RICTOR is the Ser473 kinase for Akt/PKB in 3T3-L1 adipocytes. J. Biol. Chem. 280, 40406–40416 (2005).
Viniegra, J. G. et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J. Biol. Chem. 280, 4029–4036 (2005).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292, 1728–1731 (2001).
Chen, W. S. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208 (2001).
Li, S., Edgar, D., Fassler, R., Wadsworth, W. & Yurchenco, P. D. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell 4, 613–624 (2003).
Critchley, D. R. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem. Soc. Trans. 32, 831–836 (2004).
Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003).
Dai, J. & Higgins, J. M. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle 4, 665–668 (2005).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Troussard, A. A., Tan, C., Yoganathan, T. N. & Dedhar, S. Cell–extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol. Cell. Biol. 19, 7420–7427 (1999).
Muranyi, A. et al. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem. J. 366, 211–216 (2002).
Quelo, I., Gauthier, C., Hannigan, G. E., Dedhar, S. & St-Arnaud, R. Integrin-linked kinase regulates the nuclear entry of the c-Jun coactivator α-NAC and its coactivation potency. J. Biol. Chem. 279, 43893–43899 (2004).
Deng, J. T., Sutherland, C., Brautigan, D. L., Eto, M. & Walsh, M. P. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem. J. 367, 517–524 (2002).
Erdodi, F. et al. Phosphorylation of protein phosphatase type-1 inhibitory proteins by integrin-linked kinase and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 306, 382–387 (2003).
Acknowledgements
The authors acknowledge support from a Marie Curie International Fellowship within the 6th European Community Framework Programme to K.R.L, an Erwin Schroedinger fellowship from the Austrian Science Foundation (FWF) to O.K, and the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie and the Max-Planck Society. The authors would like to thank C. Zervas and members of the Fässler laboratory for critical reading of the manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Related links
DATABASES
Flybase
Interpro
Swiss-Prot
FURTHER INFORMATION
Glossary
- Extracellular matrix
-
(ECM). A network of secreted proteins and polysaccharides that surrounds all the connective tissues and underlines all the epithelial and the endothelial sheets. It provides a physical support for tissues, as well as a sink for the storage, release and presentation of growth factors.
- Focal adhesion
-
A highly specialized cell-adhesion structure that connects actin filaments to the ECM through integrins. Immature focal adhesions are known as focal complexes, and those that are formed through interactions with fibronectin mature into structures known as fibrillar adhesions.
- Ankyrin repeat
-
A protein–protein-interaction module that consists of approximately 30 amino acids. It was first identified in the yeast cell-cycle regulator Swi6/Cdc10 and the D. melanogaster signalling protein Notch, and it was named after the cytoskeletal protein ankyrin. This motif is found in more than 1,700 different proteins.
- Pleckstrin homology (PH) domain
-
A phosphoinositide-binding motif that is composed of approximately 100 amino acids and is involved in receiving and transmitting signals at the interface between the membrane and cytosol.
- Senescent
-
Cells that are undergoing a permanent form of cell-cycle arrest that was originally described for post-proliferative primary cells in culture. Senescence can be induced by DNA damage, oxidative stress, chemotherapy and excess mitogenic stimuli, and is controlled by the tumour suppressor proteins, p53 and retioblastoma protein.
- LIM domain
-
A tandem cysteine-rich Zn2+-finger motif that mediates protein–protein interactions. It was originally identified in the transcription factors LIN11, ISL1 and MEC3.
- Calponin homology (CH) domain
-
A relatively small motif that is present in several cytoskeletal proteins and functions as an actin-binding domain, especially when they are presented in tandem.
- Small inhibitory RNA
-
(siRNA). Double-stranded RNA molecules of 21–25 nucleotides in length that are used as a viral defence mechanism and an endogenous gene-silencing mechanism from plants to humans.
- Lamellipodium
-
Dynamic actin-mediated cell-membrane protrusions at the front of spreading and migrating cells. They are essential for cell motility, phagocytosis and the development of substrate adhesions.
- RNA interference
-
(RNAi). A method to silence specific gene expression by introducing double-stranded RNA into the cell that matches the nucleotide sequence of the targeted mRNA.
- LD motif
-
Leucine-rich protein-binding sequences with the consensus sequence LDXLLXXL.
- Kindler syndrome
-
An inheritable epidermal defect that is characterized by blistering, abnormal pigmentation, fragile skin and increased cancer risk.
- Thymosins
-
A large family of small peptides that was originally identified in the thymus, but is also found in many tissues. They are divided into three main groups: α-, β-, and γ-thymosins. β-Thymosins bind globular actin to maintain a pool of actin monomers in the cell.
- Calpains
-
A superfamily of multimeric Ca2+-dependent cysteine proteases that is implicated in various cellular processes such as proliferation, differentiation and apoptosis. Deregulation of calpain activity has been implicated in various pathological conditions.
- Sarcolemma
-
The plasma membrane that encloses striated muscle fibres.
- Adherens junction
-
A highly specialized cell–cell-adhesion complex that contains cadherins and catenins, and which is connected to cytoplasmic actin filaments.
- Dominant negative
-
Introduction of an inactive mutant gene product, which interferes with the functional endogenous gene product, perhaps by competing for available accessory factors.
- Ataxia telangiectasia
-
(ATM). Autosomal recessive hereditary disease associated with DNA-repair defects and caused by mutations in the ATM (ataxia telangiectasia) gene. It is characterized by progressive cerebellar ataxia, dilation of blood vessels in the skin and eyes, chromosomal aberrations, immune dysfunction and an increased risk of cancer malignancy, particularly leukaemia and lymphoma.
- PAT phenotype
-
A broad phenotypic class of lethal mutations that affect muscle formation in C. elegans. Mutations that cause a PAT (paralyzed and arrested elongation at twofold) phenotype, affect either components of the attachment complex or essential components within the sarcomere.
- Dense bodies/M-lines
-
Focal-adhesion-like muscle-attachment structures in C. elegans. Dense bodies anchor actin filaments to the plasma membrane, and M-lines attach myosin filaments to the plasma membrane. Both structures are essential for the contractility and the maintenance of the muscles.
- Dorsal closure
-
A mid-stage developmental process that involves the movement of lateral dorsal epithelia towards the dorsal midline. This process is required for the sealing of embryonic epidermis in D. melanogaster.
- Podocyte
-
Highly specialized epithelial cells that cover the outer aspect of the glomerular basement membrane in the kidney. Mature podocytes possess a highly branched array of foot processes that are essential for glomerular filtration.
- Chondrodysplasia
-
A heterogeneous group of genetic disorders, which are characterized by abnormal skeletal morphogenesis affecting the development and growth of most skeletal elements.
- Inner cell mass
-
Inner cells of the blastocyst that retain pluripotency and give rise to all cell types of the future body.
- Blastocyst
-
An early stage of embryonic development, during which cells begin to commit to developmental lineages.
- Embryoid bodies
-
Three-dimensional spherical aggregates of differentiated cells that are derived from embryonic stem cells. The differentiation of embryoid bodies recapitulates many aspects of the early course of embryonic development in vivo.
- Basement membrane
-
Specialized extracellular matrix that first appears during the peri-implantation stage in vertebrates and during gastrulation in invertebrates.
Rights and permissions
About this article
Cite this article
Legate, K., Montañez, E., Kudlacek, O. et al. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7, 20–31 (2006). https://doi.org/10.1038/nrm1789
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm1789
This article is cited by
-
Genome-wide association studies for economically important traits in mink using copy number variation
Scientific Reports (2024)
-
Inhibition of the ILK-AKT pathway by upregulation of PARVB contributes to the cochlear cell death in Fascin2 gene knockout mice
Cell Death Discovery (2024)
-
Anoikis resistance––protagonists of breast cancer cells survive and metastasize after ECM detachment
Cell Communication and Signaling (2023)
-
Requirement for PINCH in skeletal myoblast differentiation
Cell and Tissue Research (2023)
-
Deletion of endothelial α-parvin inhibits tumour angiogenesis, reduces tumour growth and induces tumour cell apoptosis
Angiogenesis (2022)