Key Points
-
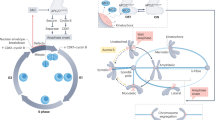
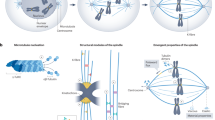
The spindle-assembly checkpoint (SAC) is a safety device that monitors the attachment of spindle microtubules to the surface of chromosome-associated structures called kinetochores. It is believed that the SAC senses the occupancy of microtubules at the surface of kinetochores, as well as the accumulation of inter-kinetochore tension when sister kinetochores are linked to opposite spindle poles.
-
The SAC is active in prometaphase during the microtubule–kinetochore attachment process, and it is downregulated when all sister chromatids have aligned to the mitotic spindle in a bipolar fashion. This triggers the loss of sister-chromatid cohesion, which initiates sister-chromatid separation at anaphase.
-
The signalling activity of the SAC in prometaphase seems to converge on the formation of at least one SAC effector, the mitotic checkpoint complex (MCC). This complex contains the SAC proteins MAD2, BUBR1 and BUB3 bound to the SAC target CDC20. This complex inhibits the activity of the anaphase-promoting complex/cyclosome (APC/C), which is required to remove sister-chromatid cohesion.
-
The way the MCC is generated from its constituent subunits is the subject of controversy. Especially controversial is the relative contribution offered by the cytosol and by kinetochores to MCC formation. There is evidence from Saccharomyces cerevisiae that the MCC can form in the absence of kinetochores. On the other hand, all SAC proteins localize to kinetochores in mitosis and it seems likely that this will contribute a mass-action effect, enhancing the rate of MCC formation.
-
A still-speculative hypothesis, the 'MAD2 template' hypothesis, proposes that the interaction of MAD2 with CDC20 follows a prion-like scenario in which an O-MAD2 conformer is primed by a kinetochore-bound C-MAD2 conformer to bind CDC20.
-
Besides the core SAC machinery, which is represented by the MCC subunits, several auxiliary functions contribute to SAC signal amplification. These include certain kinases (BUB1, BUBR1, MPS1 and Aurora B), the microtubule motor protein centromere protein E (CENP-E), and components of the ROD–ZW10–ZWILCH (RZZ) complex. p31comet and dynein, on the other hand, contribute to the downregulation of SAC signalling.
-
Subtle alterations of SAC function might cause aneuploidy and accelerate tumorigenesis. On the other hand, the SAC is an essential device in metazoans, and current evidence indicates that its functions are also essential for the survival of cancer cells. This points to the SAC as a possible target in cancer therapy.
Abstract
In eukaryotes, the spindle-assembly checkpoint (SAC) is a ubiquitous safety device that ensures the fidelity of chromosome segregation in mitosis. The SAC prevents chromosome mis-segregation and aneuploidy, and its dysfunction is implicated in tumorigenesis. Recent molecular analyses have begun to shed light on the complex interaction of the checkpoint proteins with kinetochores — structures that mediate the binding of spindle microtubules to chromosomes in mitosis. These studies are finally starting to reveal the mechanisms of checkpoint activation and silencing during mitotic progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoyt, M. A., Totis, L. & Roberts, B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 (1991).
Li, R. & Murray, A. Feedback control of mitosis in budding yeast. Cell 66, 519–531 (1991). References 1 and 2 report founding work that describes the identification of the BUB and MAD genes in S. cerevisiae.
Musacchio, A. & Hardwick, K. G. The spindle checkpoint: structural insights into dynamic signalling. Nature Rev. Mol. Cell Biol. 3, 731–741 (2002).
Taylor, S. S., Scott, M. I. & Holland, A. J. The spindle checkpoint: a quality control mechanism which ensures accurate chromosome segregation. Chromosome Res. 12, 599–616 (2004).
Hwang, L. H. et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279, 1041–1044 (1998).
Kim, S. H., Lin, D. P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006).
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001). First identification of the MCC in human cells and presentation of the 'kinetochore sensitization' model.
Fang, G., Yu, H. & Kirschner, M. W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12, 1871–1883 (1998).
Wassmann, K. & Benezra, R. Mad2 transiently associates with an APC/p55Cdc complex during mitosis. Proc. Natl Acad. Sci. USA 95, 11193–11198 (1998).
Wu, H. et al. p55CDC/hCDC20 is associated with BUBR1 and may be a downstream target of the spindle checkpoint kinase. Oncogene 19, 4557–4562 (2000).
Tang, Z., Bharadwaj, R., Li, B. & Yu, H. Mad2-independent inhibition of APC–Cdc20 by the mitotic checkpoint protein Bub1R. Dev. Cell 1, 227–237 (2001).
Shannon, K. B., Canman, J. C. & Salmon, E. D. Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell 13, 3706–3719 (2002).
Hardwick, K. G., Johnston, R. C., Smith, D. L. & Murray, A. W. MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J. Cell Biol. 148, 871–882 (2000).
Fraschini, R. et al. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 20, 6648–6659 (2001).
Millband, D. N. & Hardwick, K. G. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell. Biol. 22, 2728–2742 (2002).
Poddar, A., Stukenberg, P. T. & Burke, D. J. Two complexes of spindle checkpoint proteins containing Cdc20 and Mad2 assemble during mitosis independently of the kinetochore in Saccharomyces cerevisiae. Eukaryot. Cell 4, 867–878 (2005).
Morrow, C. J. et al. Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J. Cell Sci. 118, 3639–3652 (2005). An insightful analysis that establishes a strong link between the activity of two SAC kinases and the association of the MCC with the APC/C.
D'Angiolella, V., Mari, C., Nocera, D., Rametti, L. & Grieco, D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 17, 2520–2525 (2003).
Kallio, M. J., McCleland, M. L., Stukenberg, P. T. & Gorbsky, G. J. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 12, 900–905 (2002).
Murata-Hori, M., Tatsuka, M. & Wang, Y. -L. Probing the dynamics and functions of Aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell 13, 1099–1108 (2002).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Hauf, S. et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281–294 (2003).
Weiss, E. & Winey, M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132, 111–123 (1996).
Abrieu, A. et al. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106, 83–93 (2001).
Hardwick, K. G., Weiss, E., Luca, F. C., Winey, M. & Murray, A. W. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273, 953–956 (1996). Demonstration that the overexpression of Mps1 in S. cerevisiae is sufficient to trigger a mitotic arrest.
Chung, E. & Chen, R. -H. Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol. Biol. Cell 13, 1501–1511 (2002).
De Antoni, A. et al. The mad1/mad2 complex as a template for mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15, 214–225 (2005). Characterization of the mechanism of kinetochore recruitment of Mad2 by a Mad1–Mad2 receptor. Introduction of the 'Mad2 template' model.
Sharp-Baker, H. & Chen, R. H. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 153, 1239–1250 (2001).
Tang, Z., Shu, H., Oncel, D., Chen, S. & Yu, H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol. Cell 16, 387–397 (2004).
Karess, R. Rod–Zw10–Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 15, 386–392 (2005).
Habu, T., Kim, S. H., Weinstein, J. & Matsumoto, T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 21, 6419–6428 (2002).
Xia, G. et al. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 23, 3133–3143 (2004).
Mapelli, M. et al. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 25, 1273–1284 (2006).
Yudkovsky, Y., Shteinberg, M., Listovsky, T., Brandeis, M. & Hershko, A. Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem. Biophys. Res. Commun. 271, 299–304 (2000).
Potapova, T. A. et al. The reversibility of mitotic exit in vertebrate cells. Nature 440, 954–958 (2006).
Chung, E. & Chen, R. H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nature Cell Biol. 5, 748–753 (2003).
Minshull, J., Sun, H., Tonks, N. K. & Murray, A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79, 475–486 (1994).
Takenaka, K., Gotoh, Y. & Nishida, E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J. Cell Biol. 136, 1091–1097 (1997).
Wang, X. M., Zhai, Y. & Ferrell, J. E. Jr. A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J. Cell Biol. 137, 433–443 (1997).
Lou, Y. et al. Nek2A interacts with Mad1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J. Biol. Chem. 279, 20049–20057 (2004).
van Vugt, M. A. & Medema, R. H. Getting in and out of mitosis with Polo-like kinase-1. Oncogene 24, 2844–2859 (2005).
Abrieu, A., Kahana, J. A., Wood, K. W. & Cleveland, D. W. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell 102, 817–826 (2000).
Mao, Y., Desai, A. & Cleveland, D. W. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J. Cell Biol. 170, 873–880 (2005). Demonstration that the kinase activity of BUBR1 is controlled by CENP-E and is downregulated when CENP-E binds to microtubules. Checkpoint inactivation might require this pathway.
Howell, B. J. et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159–1172 (2001). This study identifies a role of dynein in 'stripping' off SAC and other proteins from kinetochores upon microtubule attachment, a fundamental mechanism of inactivation of the SAC signal.
Wojcik, E. et al. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nature Cell Biol. 3, 1001–1007 (2001).
Tai, C. Y., Dujardin, D. L., Faulkner, N. E. & Vallee, R. B. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J. Cell Biol. 156, 959–968 (2002).
Cleveland, D. W., Mao, Y. & Sullivan, K. F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407–421 (2003).
Maiato, H., Deluca, J., Salmon, E. D. & Earnshaw, W. C. The dynamic kinetochore–microtubule interface. J. Cell Sci. 117, 5461–5477 (2004).
Howell, B. J., Hoffman, D. B., Fang, G., Murray, A. W. & Salmon, E. D. Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J. Cell Biol. 150, 1233–1250 (2000). The first, eye-opening study to show that Mad2 cycles rapidly on and off kinetochores.
Howell, B. J. et al. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 14, 953–964 (2004). An extension of reference 50 to several other SAC proteins.
Kallio, M. J., Beardmore, V. A., Weinstein, J. & Gorbsky, G. J. Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J. Cell Biol. 158, 841–847 (2002).
Shah, J. V. et al. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 14, 942–952 (2004). An important demonstration that the kinetochore Mad2 exists in two non-exchanging pools.
Vink, M. et al. In vitro FRAP identifies the minimal requirements for Mad2 kinetochore dynamics. Curr. Biol. 16, 755–766 (2006). This study reports the reconstitution in vitro using recombinant material of faithful Mad2 kinetochore dynamics.
Hardwick, K. G. et al. Lesions in many different spindle components activate the spindle checkpoint in the budding yeast Saccharomyces cerevisiae. Genetics 152, 509–518 (1999).
Chan, G. K., Liu, S. T. & Yen, T. J. Kinetochore structure and function. Trends Cell Biol. 15, 589–598 (2005).
Irniger, S. Preventing fatal destruction: inhibitors of the anaphase-promoting complex in meiosis. Cell Cycle 5, 405–415 (2006).
McIntosh, J. R. Structural and mechanical control of mitotic progression. Cold Spring Harb. Symp. Quant. Biol. 56, 613–619 (1991).
Gorbsky, G. J. Kinetochores, microtubules and the metaphase checkpoint. Trends Cell Biol. 5, 143–148 (1995).
Pangilinan, F. & Spencer, F. Abnormal kinetochore structure activates the spindle assembly checkpoint in budding yeast. Mol. Biol. Cell 7, 1195–1208 (1996).
Wang, Y. & Burke, D. J. Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 15, 6838–6844 (1995).
Spencer, F. & Hieter, P. Centromere DNA mutations induce a mitotic delay in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 89, 8908–8912 (1992).
Goh, P. Y. & Kilmartin, J. V. NDC10: a gene involved in chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 121, 503–512 (1993).
Gardner, R. D. et al. The spindle checkpoint of the yeast Saccharomyces cerevisiae requires kinetochore function and maps to the CBF3 domain. Genetics 157, 1493–1502 (2001).
Gorbsky, G. J. & Ricketts, W. A. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J. Cell Biol. 122, 1311–1321 (1993).
Rieder, C. L., Schultz, A., Cole, R. & Sluder, G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127, 1301–1310 (1994).
Rieder, C. L., Cole, R. W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941–948 (1995). A must-read, classic, cell-biology paper showing that a single unattached kinetochore is sufficient to maintain the checkpoint signal.
Nicklas, R. B., Ward, S. C. & Gorbsky, G. J. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 130, 929–939 (1995). Another classic cell-biology study demonstrating that kinetochore chemistry is sensitive to the tension applied to kinetochores.
Taylor, S. S., Ha, E. & McKeon, F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 142, 1–11 (1998).
Taylor, S. S. & McKeon, F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89, 727–735 (1997).
Li, Y. & Benezra, R. Identification of a human mitotic checkpoint gene: hsMAD2. Science 274, 246–248 (1996).
Chen, R. H., Waters, J. C., Salmon, E. D. & Murray, A. W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science 274, 242–246 (1996).
Chen, R. H., Shevchenko, A., Mann, M. & Murray, A. W. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 143, 283–295 (1998).
Meraldi, P., Draviam, V. M. & Sorger, P. K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 7, 45–60 (2004). A careful, quantitative analysis of the effects of SAC- and kinetochore-protein depletion on anaphase timing, leading to the presentation of the 'timer' idea.
McAinsh, A. D., Meraldi, P., Draviam, V. M., Toso, A. & Sorger, P. K. The human kinetochore proteins Nnf1R and Mcm21R are required for accurate chromosome segregation. EMBO J. 25, 4033–4049 (2006).
Zhao, Y. & Chen, R. H. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr. Biol. 16, 1764–1769 (2006).
Vigneron, S. et al. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell 15, 4584–4596 (2004).
Meraldi, P., McAinsh, A. D., Rheinbay, E. & Sorger, P. K. Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol. 7, R23 (2006).
DeLuca, J. G. & Salmon, E. D. Kinetochores: if you build it, they will come. Curr. Biol. 14, R921–R923 (2004).
Deluca, J. G. et al. Kinetochore microtubule dynamics and attachment stability are regulated by hec1. Cell 127, 969–982 (2006).
Cheeseman, I. M., Chappie, J. S., Wilson-Kubalek, E. M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006). References 80 and 81 define a role for the Ndc80/HEC1 complex in microtubule binding at the kinetochore, a fundamental discovery to understand kinetochore structure and function.
Liu, S. T., Rattner, J. B., Jablonski, S. A. & Yen, T. J. Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J. Cell Biol. 175, 41–53 (2006).
Johnson, V. L., Scott, M. I., Holt, S. V., Hussein, D. & Taylor, S. S. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 117, 1577–1589 (2004).
Clute, P. & Pines, J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nature Cell Biol. 1, 82–87 (1999).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Rieder, C. L. & Maiato, H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7, 637–651 (2004).
Waters, J. C., Chen, R. H., Murray, A. W. & Salmon, E. D. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181–1191 (1998). An important study demonstrating that the localization of Mad2 to kinetochores is sensitive to microtubule attachment.
Skoufias, D. A., Andreassen, P. R., Lacroix, F. B., Wilson, L. & Margolis, R. L. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl Acad. Sci. USA 98, 4492–4497 (2001).
Nicklas, R. B. How cells get the right chromosomes. Science 275, 632–637 (1997).
Nicklas, R. B., Waters, J. C., Salmon, E. D. & Ward, S. C. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J. Cell Sci. 114, 4173–4183 (2001).
Pinsky, B. A. & Biggins, S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. (2005).
Tanaka, T. U. et al. Evidence that the Ipl1–Sli15 (Aurora kinase–INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Trends Cell Biol. 15, 486–493 (2005). An elegant set of experiments demonstrating that Aurora kinase is required for the correction of syntelic attachment.
Lampson, M. A., Renduchitala, K., Khodjakov, A. & Kapoor, T. M. Correcting improper chromosome-spindle attachments during cell division. Nature Cell Biol. 6, 232–237 (2004).
Cimini, D. & Degrassi, F. Aneuploidy: a matter of bad connections. Trends Cell Biol. 15, 442–451 (2005).
Zhou, J., Yao, J. & Joshi, H. C. Attachment and tension in the spindle assembly checkpoint. J. Cell Sci. 115, 3547–3555 (2002).
Pan, J. & Chen, R. H. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 18, 1439–1451 (2004).
Camasses, A., Bogdanova, A., Shevchenko, A. & Zachariae, W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol. Cell 12, 87–100 (2003).
Fang, G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell 13, 755–766 (2002).
Davenport, J., Harris, L. D. & Goorha, R. Spindle checkpoint function requires Mad2-dependent Cdc20 binding to the Mad3 homology domain of BubR1. Exp. Cell Res. 312, 1831–1842 (2006).
Chen, R. -H. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158, 487–496 (2002).
Kramer, E. R., Scheuringer, N., Podtelejnikov, A. V., Mann, M. & Peters, J. M. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555–1569 (2000).
Gillett, E. S., Espelin, C. W. & Sorger, P. K. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J. Cell Biol. 164, 535–546 (2004).
den Elzen, N. & Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121–136 (2001).
Geley, S. et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153, 137–148 (2001).
Doncic, A., Ben-Jacob, E. & Barkai, N. Evaluating putative mechanisms of the mitotic spindle checkpoint. Proc. Natl Acad. Sci. USA 102, 6332–6337 (2005).
Sear, R. P. & Howard, M. Modeling dual pathways for the metazoan spindle assembly checkpoint. Proc. Natl Acad. Sci. USA 103, 16758–16763 (2006).
Acquaviva, C., Herzog, F., Kraft, C. & Pines, J. The anaphase promoting complex/cyclosome is recruited to centromeres by the spindle assembly checkpoint. Nature Cell Biol. 6, 892–898 (2004).
Chan, G. K., Jablonski, S. A., Sudakin, V., Hittle, J. C. & Yen, T. J. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941–954 (1999).
Luo, X. et al. Structure of the mad2 spindle assembly checkpoint protein and its interaction with cdc20. Nature Struct. Biol. 7, 224–229 (2000).
Luo, X., Tang, Z., Rizo, J. & Yu, H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9, 59–71 (2002). A structural study showing that Mad2 undergoes a dramatic conformational change upon binding to its ligands.
Sironi, L. et al. Crystal structure of the tetrameric Mad1–Mad2 core complex: implications of a 'safety belt' binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002). Another structural study that reveals an astonishing mechanism of binding based on a comformationally mobile element of Mad2, the safety belt.
Chen, R. H., Brady, D. M., Smith, D., Murray, A. W. & Hardwick, K. G. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol. Biol. Cell 10, 2607–2618 (1999).
Luo, X. et al. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nature Struct. Mol. Biol. 11, 338–345 (2004).
Nezi, L. et al. Accumulation of Mad2:Cdc20 complex during spindle checkpoint activation requires binding of open and closed conformers of Mad2 in Saccharomyces cerevisiae. J. Cell Biol. 174, 39–51 (2006).
Nasmyth, K. How do so few control so many? Cell 120, 739–746 (2005).
Murata-Hori, M. & Wang, Y. -L. The kinase activity of Aurora B is required for kinetochore-microtubule interactions during mitosis. Curr. Biol. 12, 894–899 (2002).
Ahonen, L. J. et al. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15, 1078–1089 (2005).
Wong, O. K. & Fang, G. Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores. J. Cell Biol. 170, 709–719 (2005).
Mao, Y., Abrieu, A. & Cleveland, D. W. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell 114, 87–98 (2003).
Lampson, M. A. & Kapoor, T. M. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nature Cell Biol. 7, 93–98 (2005).
Chan, G. K., Schaar, B. T. & Yen, T. J. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J. Cell Biol. 143, 49–63 (1998).
Yao, X., Abrieu, A., Zheng, Y., Sullivan, K. F. & Cleveland, D. W. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nature Cell Biol. 2, 484–491 (2000).
Schaar, B. T., Chan, G. K., Maddox, P., Salmon, E. D. & Yen, T. J. CENP-E function at kinetochores is essential for chromosome alignment. J. Cell Biol. 139, 1373–1382 (1997).
McEwen, B. F. et al. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell 12, 2776–2789 (2001).
Weaver, B. A. et al. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 162, 551–563 (2003). An important study clarifying that CENP-E is required to sustain checkpoint signalling when there are only one or a few unattached kinetochores.
Kapoor, T. M. et al. Chromosomes can congress to the metaphase plate before biorientation. Science 311, 388–391 (2006).
Taylor, S. S., Hussein, D., Wang, Y., Elderkin, S. & Morrow, C. J. Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J. Cell Sci. 114, 4385–4395 (2001).
Warren, C. D. et al. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell 13, 3029–3041 (2002).
Meraldi, P. & Sorger, P. K. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 24, 1621–1633 (2005).
Tang, Z. et al. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell 10, 575–585 (2006).
Tang, Z., Sun, Y., Harley, S. E., Zou, H. & Yu, H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl Acad. Sci. USA 101, 18012–18017 (2004).
Watanabe, Y. Shugoshin: guardian spirit at the centromere. Curr. Opin. Cell Biol. 17, 590–595 (2005).
Vaur, S. et al. Control of Shugoshin function during fission-yeast meiosis. Curr. Biol. 15, 2263–2270 (2005).
Chen, R. H. Phosphorylation and activation of Bub1 on unattached chromosomes facilitate the spindle checkpoint. EMBO J. 23, 3113–3121 (2004).
Yamaguchi, S., Decottignies, A. & Nurse, P. Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J. 22, 1075–1087 (2003).
Yu, H. & Tang, Z. Bub1 multitasking in mitosis. Cell Cycle 4, 262–265 (2005).
Kitajima, T. S., Hauf, S., Ohsugi, M., Yamamoto, T. & Watanabe, Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 15, 353–359 (2005).
Indjeian, V. B., Stern, B. M. & Murray, A. W. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307, 130–133 (2005).
Liu, S. -T. et al. Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol. Biol. Cell 14, 1638–1651 (2003).
Martin-Lluesma, S., Stucke, V. M. & Nigg, E. A. Role of hec1 in spindle checkpoint signaling and kinetochore recruitment of mad1/mad2. Science 297, 2267–2270 (2002).
Campbell, M. S., Chan, G. K. & Yen, T. J. Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J. Cell Sci. 114, 953–963 (2001).
Dasso, M. Ran at kinetochores. Biochem. Soc. Trans. 34, 711–715 (2006).
Vader, G., Medema, R. H. & Lens, S. M. The chromosomal passenger complex: guiding Aurora-B through mitosis. J. Cell Biol. 173, 833–837 (2006).
Carmena, M. & Earnshaw, W. C. The cellular geography of aurora kinases. Nature Rev. Mol. Cell Biol. 4, 842–854 (2003).
Biggins, S. & Murray, A. W. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15, 3118–3129 (2001).
Pinsky, B. A., Kung, C., Shokat, K. M. & Biggins, S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nature Cell Biol. 8, 78–83 (2006).
Buffin, E., Lefebvre, C., Huang, J., Gagou, M. E. & Karess, R. E. Recruitment of mad2 to the kinetochore requires the rod/zw10 complex. Curr. Biol. 15, 856–861 (2005).
Liu, S. T. et al. Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nature Cell Biol. 5, 341–345 (2003).
DeLuca, J. G. et al. Nuf2 and hec1 are required for retention of the checkpoint proteins mad1 and mad2 to kinetochores. Curr. Biol. 13, 2103–2109 (2003).
Kops, G. J. et al. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169, 49–60 (2005).
Wang, H. et al. Human zwint-1 specifies localization of zeste white 10 to kinetochores and is essential for mitotic checkpoint signaling. J. Biol. Chem. 279, 54590–54598 (2004).
Lin, Y. T., Chen, Y., Wu, G. & Lee, W. H. Hec1 sequentially recruits Zwint-1 and ZW10 to kinetochores for faithful chromosome segregation and spindle checkpoint control. Oncogene 25, 6901–6914 (2006).
DeLuca, J. G., Moree, B., Hickey, J. M., Kilmartin, J. V. & Salmon, E. D. hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J. Cell Biol. 159, 549–555 (2002).
King, J. M., Hays, T. S. & Nicklas, R. B. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J. Cell Biol. 151, 739–748 (2000).
Hoffman, D. B., Pearson, C. G., Yen, T. J., Howell, B. J. & Salmon, E. D. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell 12, 1995–2009 (2001).
Basto, R. et al. In vivo dynamics of the rough deal checkpoint protein during Drosophila mitosis. Curr. Biol. 14, 56–61 (2004).
Tong, A. H. et al. Global mapping of the yeast genetic interaction network. Science 303, 808–813 (2004).
Wassmann, K., Liberal, V. & Benezra, R. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 22, 797–806 (2003).
de Gramont, A., Ganier, O. & Cohen-Fix, O. Before and after the spindle assembly chekpoint. Cell Cycle 5, 2168–2171 (2006).
Palframan, W. J., Meehl, J. B., Jaspersen, S. L., Winey, M. & Murray, A. W. Anaphase inactivation of the spindle checkpoint. Science 313, 680–684 (2006).
Qi, W. & Yu, H. KEN-box-dependent degradation of the bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome. J. Biol. Chem. 282, 3672–3679 (2007).
Brito, D. A. & Rieder, C. L. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 16, 1194–1200 (2006).
McCleland, M. L. et al. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101–114 (2003).
Kops, G. J., Weaver, B. A. & Cleveland, D. W. On the road to cancer: aneuploidy and the mitotic checkpoint. Nature Rev. Cancer 5, 773–785 (2005). This review discusses that systematic SAC inactivation leads to lethality of cancer cells, suggesting that the SAC might be regarded as a possible target for anti-tumour therapy.
Tighe, A., Johnson, V. L., Albertella, M. & Taylor, S. S. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2, 609–614 (2001).
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007).
Kops, G. J., Foltz, D. R. & Cleveland, D. W. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl Acad. Sci. USA 101, 8699–8704 (2004).
Weaver, B. A., Silk, A. D., Montagna, C., Verdier-Pinard, P. & Cleveland, D. W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007).
Girdler, F. et al. Validating Aurora B as an anti-cancer drug target. J. Cell Sci. 119, 3664–3675 (2006).
Keen, N. & Taylor, S. Aurora-kinase inhibitors as anticancer agents. Nature Rev. Cancer 4, 927–936 (2004).
Wang, X. et al. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. J. Biol. Chem. 276, 26559–26567 (2001).
Okada, M. et al. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nature Cell Biol. 8, 446–457 (2006).
Foltz, D. R. et al. The human CENP-A centromeric nucleosome-associated complex. Nature Cell Biol 8, 458–469 (2006).
Cimini, D., Wan, X., Hirel, C. B. & Salmon, E. D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711–1718 (2006).
Knowlton, A. L., Lan, W. & Stukenberg, P. T. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 16, 1705–1710 (2006).
Tanaka, T. U., Stark, M. J. & Tanaka, K. Kinetochore capture and bi-orientation on the mitotic spindle. Nature Rev. Mol. Cell Biol. 6, 929–942 (2005).
Acknowledgements
We thank S. Piatti and members of the Musacchio and Salmon laboratories for critical reading of the manuscript and helpful discussions. Research in the Musacchio laboratory is funded by the Association for International Cancer Research (AICR), the Telethon Foundation, the EU FP6 programme contracts 3D-Repertoire and Mitocheck, the Italian Association for Cancer Research (AIRC), the Fondo di Investimento per la Ricerca di Base (FIRB), the Italian Ministry of Health, the Fondazione Cariplo and the Human Frontier Science Program. Research in the Salmon laboratory is funded by grants from the US National Institutes of Health and the Human Frontier Science Program. We deeply apologize to all colleagues whose work could not be cited owing to space limitations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Anaphase-promoting complex/cyclosome
-
(APC/C). A multiprotein complex with ubiquitin-ligase activity that is responsible for the ubiquitylation of several key cell-cycle regulators, including cyclin B and securin. Also known as the cyclosome.
- 26S proteasome
-
A multiprotein complex endowed with protease activity. It is responsible for the proteolytic degradation of substrates tagged by polyubiquitin chains, including those created by the APC/C.
- Mitotic checkpoint complex
-
(MCC). A complex that contains the APC/C activator CDC20 and the spindle-assembly checkpoint proteins MAD2, BUBR1/Mad3 and BUB3. The MCC is regarded as the effector of the spindle-assembly checkpoint.
- Kinetochore
-
A large protein assembly that mediates the attachment of chromosomes to spindle microtubules. Kinetochores assemble, specifically during mitosis, around specialized chromosomal regions known as centromeres, and disassemble at the end of mitosis.
- Monotelic attachment
-
A condition in which only one sister kinetochore in a pair of sister kinetochores is attached to kinetochore microtubules. Monotelic attachment is a normal stage during the process of microtubule–kinetochore attachment and chromosome bi-orientation in prometaphase. The unattached kinetochore of a mono-orientated chromosome is a potent checkpoint signal.
- Histone variant
-
A non-allelic variant of the histone proteins that has specific expression and localization patterns.
- Syntelic attachment
-
A type of incorrect microtubule–kinetochore attachment in which both sister kinetochores become attached to microtubules from the same spindle pole. Syntelically attached kinetochores often reside near a pole and do not congress to the spindle equator. The correction of syntelic attachment requires the Aurora-B/Ipl1 kinase.
- Merotelic attachment
-
A type of incorrect microtubule–kinetochore attachment in which a kinetochore becomes attached to microtubules from both spindle poles. Merotelic attachment by one sister kinetochore does not prevent chromosome bi-orientation by monotelic attachment of the other sister kinetochore and it does not activate the spindle-assembly checkpoint. However, a mechanism of correction based on the Aurora-B/Ipl1 kinase exists.
- KEN-box motif
-
A sequence motif (KENXXXN) that is present in several substrates of the APC/C.
- Fluorescence recovery after photobleaching
-
(FRAP). An imaging technique that measures the kinetics and extent of fluorescence recovery in small volumes that have been subjected to a short high-energy laser pulse to irreversibly bleach a fluorophore. The recovery curves measure labelled-protein diffusion rates in the cytosol and dissociation rate constants from binding sites.
- Chromosomal passenger complex
-
(CPC). A protein complex that shares a characteristic pattern of association with chromatin in prophase, centromeres in metaphase and early anaphase, and the midzone and midbody in late anaphase and telophase, respectively.
Rights and permissions
About this article
Cite this article
Musacchio, A., Salmon, E. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8, 379–393 (2007). https://doi.org/10.1038/nrm2163
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2163
This article is cited by
-
MAPK-dependent control of mitotic progression in S. pombe
BMC Biology (2024)
-
RNA sequencing and target long-read sequencing reveal an intronic transposon insertion causing aberrant splicing
Journal of Human Genetics (2024)
-
Non-reciprocal multifarious self-organization
Nature Nanotechnology (2023)
-
BAP1 loss induces mitotic defects in mesothelioma cells through BRCA1-dependent and independent mechanisms
Oncogene (2023)
-
Schizophrenia-associated Mitotic Arrest Deficient-1 (MAD1) regulates the polarity of migrating neurons in the developing neocortex
Molecular Psychiatry (2023)