Key Points
-
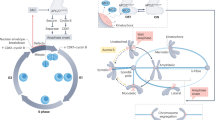
The completion of mitosis is governed by the dephosphorylation of cyclin-dependent kinase (Cdk) substrates and by the ubiquitination of anaphase-promoting complex (APC) substrates. The order in which these substrates are modified helps to establish the order of late mitotic events.
-
The order of Cdk-substrate dephosphorylation depends in part on the timing of Cdk inactivation, which is determined primarily by the order of destruction of different cyclin types.
-
Cdk-substrate dephosphorylation also depends on phosphatases, of which the best-known example is Cdc14 of budding yeast. The two-step Cdc14 activation process helps to determine the sequence of Cdk-substrate dephosphorylation.
-
APC activity and substrate specificity depend on the interaction of APC with the activating subunits Cdc20 and Cdh1. The sequential activation of APC by these subunits — Cdc20 first, followed by Cdh1 — determines the order of APC-substrate modification.
-
The spindle-assembly checkpoint contributes to APC-substrate ordering by delaying the ubiquitination of some substrates until chromosomes are properly attached to the mitotic spindle.
-
APC-substrate ordering also depends on the intrinsic differences in the activity of the APC towards different substrates.
Abstract
The final stages of mitosis begin in anaphase, when the mitotic spindle segregates the duplicated chromosomes. Mitotic exit is then completed by disassembly of the spindle and packaging of chromosomes into daughter nuclei. The successful completion of mitosis requires that these events occur in a strict order. Two main mechanisms govern progression through late mitosis: dephosphorylation of cyclin-dependent kinase (Cdk) substrates and destruction of the substrates of the anaphase-promoting complex (APC). Here, we discuss the hypothesis that the order of late mitotic events depends, at least in part, on the order in which different Cdk and APC substrates are dephosphorylated or destroyed, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Morgan, D. O. The Cell Cycle: Principles of Control (New Science Press, London, 2007).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006).
Thornton, B. R. & Toczyski, D. P. Precise destruction: an emerging picture of the APC. Genes Dev. 20, 3069–3078 (2006).
Furuno, N., den Elzen, N. & Pines, J. Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 147, 295–306 (1999).
Gong, D. et al. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr. Biol. 17, 85–91 (2007).
den Elzen, N. & Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121–136 (2001).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Sigrist, S., Jacobs, J., Stratmann, R. & Lehner, C. F. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B, and B3. EMBO J. 14, 4827–4838 (1995).
Jacobs, H. W., Knoblich, J. A. & Lehner, C. F. Drosophila cyclin B3 is required for female fertility and is dispensable for mitosis like cyclin B. Genes Dev. 12, 3741–3751 (1998).
Bloom, J. & Cross, F. R. Multiple levels of cyclin specificity in cell-cycle control. Nature Rev. Mol. Cell Biol. 8, 149–160 (2007).
Parry, D. H. & O'Farrell, P. H. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 11, 671–683 (2001). Evidence that the timing of destruction of distinct cyclins by the APC helps to order late mitotic events.
Peeper, D. S. et al. A- and B-type cyclins differentially modulate substrate specificity of cyclin-CDK complexes. EMBO J. 12, 1947–1954 (1993).
Brown, N. R., Noble, M. E., Endicott, J. A. & Johnson, L. N. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nature Cell Biol. 1, 438–443 (1999).
Loog, M. & Morgan, D. O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434, 104–108 (2005).
Stegmeier, F. & Amon, A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38, 203–232 (2004).
D'Amours, D. & Amon, A. At the interface between signaling and executing anaphase — Cdc14 and the FEAR network. Genes Dev. 18, 2581–2595 (2004).
Shou, W. et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233–244 (1999).
Visintin, R., Hwang, E. S. & Amon, A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398, 818–823 (1999).
Stegmeier, F., Visintin, R. & Amon, A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108, 207–220 (2002).
Queralt, E., Lehane, C., Novak, B. & Uhlmann, F. Downregulation of PP2ACdc55 phosphatase by separase initiates mitotic exit in budding yeast. Cell 125, 719–732 (2006). Evidence that separase promotes Cdc14 activation during early anaphase by blocking the actions of the phosphatase PP2A.
Jaspersen, S. L., Charles, J. F., Tinker-Kulberg, R. L. & Morgan, D. O. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol. Biol. Cell 9, 2803–2817 (1998).
D'Amours, D., Stegmeier, F. & Amon, A. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell 117, 455–469 (2004).
Sullivan, M., Higuchi, T., Katis, V. L. & Uhlmann, F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell 117, 471–482 (2004).
Ross, K. E. & Cohen-Fix, O. A role for the FEAR pathway in nuclear positioning during anaphase. Dev. Cell 6, 729–735 (2004).
Higuchi, T. & Uhlmann, F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature 433, 171–176 (2005). Demonstration that Cdc14 activation during early anaphase promotes normal anaphase spindle and chromosome behaviours.
Pereira, G. & Schiebel, E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 302, 2120–2124 (2003).
Woodbury, E. L. & Morgan, D. O. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nature Cell Biol. 9, 106–112 (2007).
Khmelinskii, A., Lawrence, C., Roostalu, J. & Schiebel, E. Cdc14-regulated midzone assembly controls anaphase B. J. Cell Biol. 177, 981–993 (2007).
Trautmann, S. & McCollum, D. Cell cycle: new functions for Cdc14 family phosphatases. Curr. Biol. 12, R733–R735 (2002).
Stemmann, O., Zou, H., Gerber, S. A., Gygi, S. P. & Kirschner, M. W. Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726 (2001).
Geley, S. et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153, 137–148 (2001).
Gorr, I. H., Boos, D. & Stemmann, O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19, 135–141 (2005).
Chang, D. C., Xu, N. & Luo, K. Q. Degradation of cyclin B is required for the onset of anaphase in mammalian cells. J. Biol. Chem. 278, 37865–37873 (2003).
Herbert, M. et al. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nature Cell Biol. 5, 1023–1025 (2003).
Parry, D. H., Hickson, G. R. & O'Farrell, P. H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 13, 647–653 (2003).
Wheatley, S. P. et al. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 138, 385–393 (1997).
Zhu, C., Lau, E., Schwarzenbacher, R., Bossy-Wetzel, E. & Jiang, W. Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc. Natl Acad. Sci. USA 103, 6196–6201 (2006).
Jaspersen, S. L., Charles, J. F. & Morgan, D. O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9, 227–236 (1999).
Zachariae, W., Schwab, M., Nasmyth, K. & Seufert, W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282, 1721–1724 (1998).
Nash, P. et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414, 514–521 (2001).
Verma, R. et al. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science 278, 455–460 (1997).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998).
Moll, T., Tebb, G., Surana, U., Robitsch, H. & Nasmyth, K. The role of phosphorylation and the CDC28 protein kinase in the cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell 66, 743–758 (1991).
Jaquenoud, M., van Drogen, F. & Peter, M. Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/CCdh1. EMBO J. 21, 6515–6526 (2002).
Bembenek, J. et al. Crm1-mediated nuclear export of Cdc14 is required for the completion of cytokinesis in budding yeast. Cell Cycle 4, 961–971 (2005).
King, R. W., Glotzer, M. & Kirschner, M. W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell 7, 1343–1357 (1996).
Pfleger, C. M. & Kirschner, M. W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–665 (2000).
Burton, J. L., Tsakraklides, V. & Solomon, M. J. Assembly of an APC–Cdh1–substrate complex is stimulated by engagement of a destruction box. Mol. Cell 18, 533–542 (2005).
Kraft, C., Vodermaier, H. C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell 18, 543–553 (2005).
Yamano, H., Gannon, J., Mahbubani, H. & Hunt, T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol. Cell 13, 137–147 (2004).
Carroll, C. W., Enquist-Newman, M. & Morgan, D. O. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 15, 11–18 (2005).
Hildebrandt, E. R. & Hoyt, M. A. Cell cycle-dependent degradation of the Saccharomyces cerevisiae spindle motor Cin8p requires APCCdh1 and a bipartite destruction sequence. Mol. Biol. Cell 12, 3402–3416 (2001).
Castro, A., Vigneron, S., Bernis, C., Labbe, J. C. & Lorca, T. Xkid is degraded in a D-box, KEN-box, and A-box-independent pathway. Mol. Cell Biol. 23, 4126–4138 (2003).
Araki, M., Wharton, R. P., Tang, Z., Yu, H. & Asano, M. Degradation of origin recognition complex large subunit by the anaphase-promoting complex in Drosophila. EMBO J. 22, 6115–6126 (2003).
Littlepage, L. E. & Ruderman, J. V. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 16, 2274–2285 (2002).
Sullivan, M. & Morgan, D. O. A novel destruction sequence targets the meiotic regulator Spo13 for anaphase-promoting complex-dependent degradation in anaphase I. J. Biol. Chem. 282, 19710–19715 (2007).
Yeong, F. M., Lim, H. H., Padmashree, C. G. & Surana, U. Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28–Clb2 mitotic kinase and the role of Cdc20. Mol. Cell 5, 501–511 (2000).
Wäsch, R. & Cross, F. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418, 556–562 (2002).
Eluere, R. et al. Compartmentalization of the functions and regulation of the mitotic cyclin Clb2 in S. cerevisiae. J. Cell Sci. 120, 702–711 (2007).
Hayes, M. J. et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nature Cell Biol. 8, 607–614 (2006). The authors suggest that certain APC substrates, such as Nek2A, can bind to the APC independently of Cdc20, triggering their destruction before metaphase.
Gabellini, D. et al. Early mitotic degradation of the homeoprotein HOXC10 is potentially linked to cell cycle progression. EMBO J. 22, 3715–3724 (2003).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 8, 379–393 (2007).
Vodermaier, H. C., Gieffers, C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13, 1459–1468 (2003).
Lindon, C. & Pines, J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 164, 233–241 (2004). Evidence for ordered proteolysis of APCCdh1 substrates during anaphase.
Pines, J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 (2006).
Rape, M., Reddy, S. K. & Kirschner, M. W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 (2006). Biochemical evidence that ordered APC-substrate degradation is determined in part by the affinity of the APC for its substrates.
Mailand, N. & Diffley, J. F. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell 122, 915–926 (2005).
Huang, J. & Raff, J. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18, 2184–2195 (1999).
Thornton, B. R. & Toczyski, D. P. Securin and B-cyclin/CDK are the only essential targets of the APC. Nature Cell Biol. 5, 1090–1094 (2003).
Juang, Y.-L. et al. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science 275, 1311–1314 (1997).
Ubersax, J. A. et al. Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 (2003).
Kerscher, O., Felberbaum, R. & Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22, 159–180 (2006).
Pickart, C. M. & Eddins, M. J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695, 55–72 (2004).
Kirkpatrick, D. S. et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biol. 8, 700–710 (2006).
Rodrigo-Brenni, M. & Morgan, D. O. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell 130, 127–139 (2007).
Thrower, J. S., Hoffman, L., Rechsteiner, M. & Pickart, C. M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102 (2000).
Carroll, C. W. & Morgan, D. O. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nature Cell Biol. 4, 880–887 (2002).
Eletr, Z. M., Huang, D. T., Duda, D. M., Schulman, B. A. & Kuhlman, B. E2 conjugating enzymes must disengage from their E1 enzymes before E3-dependent ubiquitin and ubiquitin-like transfer. Nature Struct. Mol. Biol. 12, 933–934 (2005).
Thornton, B. R. et al. An architectural map of the anaphase-promoting complex. Genes Dev. 20, 449–460 (2006).
Azzam, R. et al. Phosphorylation by cyclin B–Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305, 516–519 (2004). Evidence that Cdk-dependent phosphorylation of Net1 promotes the release of the phosphatase Cdc14 during anaphase.
Hu, F. et al. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107, 655–665 (2001).
Jaspersen, S. L. & Morgan, D. O. Cdc14 activates Cdc15 to promote mitotic exit in budding yeast. Curr. Biol. 10, 615–618 (2000).
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Hydrophobic patch
-
A short stretch of amino acids on the surface of some cyclins near the Cdk active site. It interacts with RXL motifs on cyclin-specific Cdk substrates or inhibitors.
- RXL motif
-
A degenerate sequence motif on some Cdk substrates and inhibitors. It interacts with the hydrophobic patch region of specific cyclins.
- Kinetochore
-
A large protein structure that assembles on the chromosome and mediates the attachment of the chromosome to microtubules of the mitotic spindle.
- Spindle midzone
-
The region at the equator of the mitotic spindle where interpolar microtubules overlap. During anaphase, this region helps to organize proteins that govern anaphase spindle behaviour and that control the initiation of cytokinesis.
- 26S proteasome
-
A large protease complex that binds polyubiquitinated proteins and degrades them.
- Destruction box
-
(D-box). A degenerate sequence motif (RXXLXXXXN) that is in most APC targets. The D-box mediates an interaction with APC activator subunits and is required for target destruction.
- KEN box
-
A degenerate sequence motif (KENXXXN) that is in some APC targets. The KEN box mediates an interaction with the APC activator Cdh1.
- Polo-like kinase-1
-
(Plk1). A protein kinase that is activated during early mitosis and that helps to promote certain mitotic events, such as spindle assembly.
- Aurora kinases A and B
-
Related protein kinases that are activated during early mitosis and govern spindle assembly, chromosome attachment to kinetochores and other mitotic processes.
- Spindle-assembly checkpoint
-
A regulatory system that monitors chromosome attachment to the mitotic spindle and delays APC activation until all chromosomes are correctly bi-orientated.
Rights and permissions
About this article
Cite this article
Sullivan, M., Morgan, D. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol 8, 894–903 (2007). https://doi.org/10.1038/nrm2276
Issue Date:
DOI: https://doi.org/10.1038/nrm2276
This article is cited by
-
Functional analysis of recurrent CDC20 promoter variants in human melanoma
Communications Biology (2023)
-
Methylation-dependent and -independent roles of EZH2 synergize in CDCA8 activation in prostate cancer
Oncogene (2022)
-
Fine-tuning cell organelle dynamics during mitosis by small GTPases
Frontiers of Medicine (2022)
-
Protein phosphatase 1 regulates atypical mitotic and meiotic division in Plasmodium sexual stages
Communications Biology (2021)
-
A cyclin-dependent kinase, CDK11/p58, represses cap-dependent translation during mitosis
Cellular and Molecular Life Sciences (2020)