Key Points
-
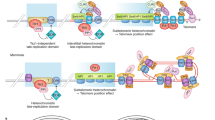
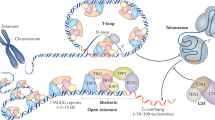
Telomeric proteins control telomere length and telomere integrity. The six bona fide telomeric binding proteins form shelterin, a complex that maintains chromosome end integrity.
-
Telomere dysfunction can be caused by loss of telomeric repeats or by loss of protective features, both of which are essential for telomere function.
-
Functional telomeres interact with the DNA damage machinery, but the machinery is prevented from processing these ends. Dysfunctional telomeres are recognized as damage and repaired.
-
Repair of dysfunctional telomeres by fusion propels cells into breakage–fusion–bridge cycles, resulting in unequal distribution of genetic material into daughter cells and, therefore, genome instability.
-
Telomere dysfunction and the failure to maintain telomere length is emerging as being the cause of several diseases.
Abstract
The natural ends of linear chromosomes require unique genetic and structural adaptations to facilitate the protection of genetic material. This is achieved by the sequestration of the telomeric sequence into a protective nucleoprotein cap that masks the ends from constitutive exposure to the DNA damage response machinery. When telomeres are unmasked, genome instability arises. Balancing capping requirements with telomere replication and the enzymatic processing steps that are obligatory for telomere function is a complex problem. Telomeric proteins and their interacting factors create an environment at chromosome ends that inhibits DNA repair; however, the repair machinery is essential for proper telomere function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Verdun, R. E. & Karlseder, J. Replication and protection of telomeres. Nature 447, 924–931 (2007).
Watson, J. D. Origin of concatemeric T7 DNA. Nature New Biol. 239, 197–201 (1972).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999). This manuscript details the finding that telomeres can form a looped structure that could distinguish natural chromosome ends form DNA breaks.
Oganesian, L. & Bryan, T. M. Physiological relevance of telomeric G-quadruplex formation: a potential drug target. Bioessays 29, 155–165 (2007).
Rhodes, D. & Giraldo, R. Telomere structure and function. Curr. Opin. Struct. Biol. 5, 311–322 (1995).
Cacchione, S., Cerone, M. A. & Savino, M. In vitro low propensity to form nucleosomes of four telomeric sequences. FEBS Lett. 400, 37–41 (1997).
Blasco, M. A. The epigenetic regulation of mammalian telomeres. Nature Rev. Genet. 8, 299–309 (2007). This publication summarizes the current knowledge of histone and DNA modifications at telomeres.
Sfeir, A. et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138, 90–103 (2009).
Martinez, P. et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 23, 2060–2075 (2009).
Raghuraman, M. K. et al. Replication dynamics of the yeast genome. Science 294, 115–121 (2001).
Palm, W. & de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 (2008).
Bianchi, A., Smith, S., Chong, L., Elias, P. & de Lange, T. TRF1 is a dimer and bends telomeric DNA. EMBO J. 16, 1785–1794 (1997).
Griffith, J., Bianchi, A. & de Lange, T. TRF1 promotes parallel pairing of telomeric tracts in vitro. J. Mol. Biol. 278, 79–88 (1998).
Amiard, S. et al. A topological mechanism for TRF2-enhanced strand invasion. Nature Struct. Mol. Biol. 14, 147–154 (2007).
de Lange, T. Protection of mammalian telomeres. Oncogene 21, 532–540 (2002).
Stansel, R. M., de Lange, T. & Griffith, J. D. T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang. EMBO J. 20, 5532–5540 (2001).
Denchi, E. L. & de Lange, T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071 (2007). This manuscript highlights the control of the ATM- and ATR-dependent DDR pathways by distinct shelterin components.
Smogorzewska, A., Karlseder, J., Holtgreve-Grez, H., Jauch, A. & de Lange, T. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 12, 1635–1644 (2002).
Benetti, R., Schoeftner, S., Munoz, P. & Blasco, M. A. Role of TRF2 in the assembly of telomeric chromatin. Cell Cycle 7, 3461–3468 (2008).
Li, B., Oestreich, S. & de Lange, T. Identification of human RAP1: implications for telomere evolution. Cell 101, 471–483 (2000).
Bae, N. S. & Baumann, P. A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol. Cell 26, 323–334 (2007).
Sarthy, J., Bae, N. S., Scrafford, J. & Baumann, P. Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J. 28, 3390–3399 (2009).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 424, 1013–1018 (2003).
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007).
Wang, F. et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Dejardin, J. & Kingston, R. E. Purification of proteins associated with specific genomic loci. Cell 136, 175–186 (2009).
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005).
Smogorzewska, A. & de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73, 177–208 (2004).
Park, J. I. et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72 (2009).
Venteicher, A. S., Meng, Z., Mason, P. J., Veenstra, T. D. & Artandi, S. E. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell 132, 945–957 (2008). This manuscript describes the essential role of ATPases in assembly of the functional telomerase complex.
Venteicher, A. S. et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 323, 644–648 (2009). This publication shows the requirement of the telomerase complex to pass through the Cajal bodies for assembly.
Luke, B. et al. The Rat1p 5' to 3' exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell 32, 465–477 (2008).
Azzalin, C. M., Reichenbach, P., Khoriauli, L., Giulotto, E. & Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318, 798–801 (2007).
Schoeftner, S. & Blasco, M. A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nature Cell Biol. 10, 228–236 (2008). References 33 and 34 recognize that telomeres can be transcribed as long repeat containing RNAs.
Luke, B. & Lingner, J. TERRA: telomeric repeat-containing RNA. EMBO J. 28, 2503–2510 (2009).
Deng, Z., Norseen, J., Wiedmer, A., Riethman, H. & Lieberman, P. M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell 35, 403–413 (2009).
Schoeftner, S. & Blasco, M. A. Chromatin regulation and non-coding RNAs at mammalian telomeres. Semin. Cell Dev. Biol. 6 Oct 2009 (doi:10.1016/j.semcdb.2009.09.015).
Makarov, V. L., Hirose, Y. & Langmore, J. P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666 (1997).
McElligott, R. & Wellinger, R. J. The terminal DNA structure of mammalian chromosomes. EMBO J. 16, 3705–3714 (1997).
Wright, W. E., Tesmer, V. M., Huffman, K. E., Levene, S. D. & Shay, J. W. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 11, 2801–2809 (1997).
Lundblad, V. & Szostak, J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57, 633–643 (1989).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Meyerson, M. et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 (1997). References 42 and 43 describe the isolation and characterization of the catalytic subunit of human telomerase.
Morales, C. P. et al. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nature Genet. 21, 115–118 (1999).
Tomas-Loba, A. et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135, 609–622 (2008). This manuscript analyses the effects of telomerase expression on the lifespan of mice rendered cancer resistant by the overexpression of tumour suppressors.
Abdallah, P. et al. A two-step model for senescence triggered by a single critically short telomere. Nature Cell Biol. 11, 988–993 (2009).
Crabbe, L., Verdun, R. E., Haggblom, C. I. & Karlseder, J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306, 1951–1953 (2004).
Karlseder, J., Smogorzewska, A. & de Lange, T. Senescence induced by altered telomere state, not telomere loss. Science 295, 2446–2449 (2002).
Xu, L. & Blackburn, E. H. Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol. Cell 28, 315–327 (2007).
Aguilaniu, H., Gustafsson, L., Rigoulet, M. & Nystrom, T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299, 1751–1753 (2003).
D'Angelo, M. A., Raices, M., Panowski, S. H. & Hetzer, M. W. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136, 284–295 (2009).
Ouellette, M. M., McDaniel, L. D., Wright, W. E., Shay, J. W. & Schultz, R. A. The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum. Mol. Genet. 9, 403–411 (2000).
Wright, W. E. & Shay, J. W. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr. Opin. Genet. Dev. 11, 98–103 (2001).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998). This manuscript recognizes the protective role of TRF2 at mammalian telomeres.
Karlseder, J., Broccoli, D., Dai, Y., Hardy, S. & de Lange, T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283, 1321–1325 (1999).
Lazzerini Denchi, E., Celli, G. & de Lange, T. Hepatocytes with extensive telomere deprotection and fusion remain viable and regenerate liver mass through endoreduplication. Genes Dev. 20, 2648–2653 (2006).
Celli, G. B. & de Lange, T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nature Cell Biol. 7, 712–718 (2005).
Baumann, P. & Cech, T. R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001). This manuscript describes the isolation and characterization of the single-stranded telomeric DNA binding factor POT1.
Hockemeyer, D., Daniels, J. P., Takai, H. & de Lange, T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell 126, 63–77 (2006).
Wu, L. et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126, 49–62 (2006).
Dimitrova, N., Chen, Y. C., Spector, D. L. & de Lange, T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456, 524–528 (2008).
Dimitrova, N. & de Lange, T. Cell cycle dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of NHEJ in G1 and resection-mediated inhibition of NHEJ in G2. Mol. Cell Biol. 29, 5552–5563 (2009).
Takai, H., Smogorzewska, A. & de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 (2003).
Bartek, J., Bartkova, J. & Lukas, J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 26, 7773–7779 (2007).
Bartek, J., Lukas, J. & Bartkova, J. DNA damage response as an anti-cancer barrier: damage threshold and the concept of 'conditional haploinsufficiency'. Cell Cycle 6, 2344–2347 (2007).
Wang, R. C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004).
Poulet, A. et al. TRF2 promotes, remodels and protects telomeric Holliday junctions. EMBO J. 28, 641–651 (2009).
Nakamura, T. M., Moser, B. A. & Russell, P. Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics 161, 1437–1452 (2002).
Nugent, C. I. et al. Telomere maintenance is dependent on activities required for end repair of double-strand breaks. Curr. Biol. 8, 657–660 (1998).
Takata, H., Kanoh, Y., Gunge, N., Shirahige, K. & Matsuura, A. Reciprocal association of the budding yeast ATM-related proteins Tel1 and Mec1 with telomeres in vivo. Mol. Cell 14, 515–522 (2004).
Tsukamoto, Y., Taggart, A. K. & Zakian, V. A. The role of the Mre11-Rad50-Xrs2 complex in telomerase- mediated lengthening of Saccharomyces cerevisiae telomeres. Curr. Biol. 11, 1328–1335 (2001).
Vaziri, H. et al. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 16, 6018–6033 (1997).
Ranganathan, V. et al. Rescue of a telomere length defect of Nijmegen breakage syndrome cells requires NBS and telomerase catalytic subunit. Curr. Biol. 11, 962–966 (2001).
Zhu, X. D., Kuster, B., Mann, M., Petrini, J. H. & de Lange, T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nature Genet. 25, 347–352 (2000).
Verdun, R. E., Crabbe, L., Haggblom, C. & Karlseder, J. Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol. Cell 20, 551–561 (2005).
Bradshaw, P. S., Stavropoulos, D. J. & Meyn, M. S. Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nature Genet. 37, 193–197 (2005).
Karlseder, J. et al. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2, E240 (2004).
Deng, Y., Guo, X., Ferguson, D. O. & Chang, S. Multiple roles for MRE11 at uncapped telomeres. Nature 460, 914–918 (2009).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Benetti, R., Garcia-Cao, M. & Blasco, M. A. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nature Genet. 39, 243–250 (2007).
Benetti, R. et al. Suv4–20h deficiency results in telomere elongation and derepression of telomere recombination. J. Cell Biol. 178, 925–936 (2007).
Garcia-Cao, M., O'Sullivan, R., Peters, A. H., Jenuwein, T. & Blasco, M. A. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nature Genet. 36, 94–99 (2004).
Gonzalo, S. et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nature Cell Biol. 8, 416–424 (2006).
McClintock, B. The fusion of broken ends of sister half chromatids following chromatid breakage at meiotic anaphase. Mo. Agric. Exp. Stn. Res. Bull. 290, 1–48 (1938).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997). This manuscript is the first one to describe the effects of telomerase inhibition on a chromosome and organism level in mammals.
Shay, J. W. & Wright, W. E. Telomerase activity in human cancer. Curr. Opin. Oncol. 8, 66–71 (1996).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000). This paper shows that telomere dysfunction leads to the development of epithelial cancers in ageing mice that lack p53.
Artandi, S. E. & DePinho, R. A. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr. Opin. Genet. Dev. 10, 39–46 (2000).
Matsutani, N. et al. Expression of telomeric repeat binding factor 1 and 2 and TRF1-interacting nuclear protein 2 in human gastric carcinomas. Int. J. Oncol. 19, 507–512 (2001).
Oh, B. K., Kim, Y. J., Park, C. & Park, Y. N. Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am. J. Pathol. 166, 73–80 (2005).
Blanco, R., Munoz, P., Flores, J. M., Klatt, P. & Blasco, M. A. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 21, 206–220 (2007).
Munoz, P., Blanco, R., Flores, J. M. & Blasco, M. A. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nature Genet. 37, 1063–1071 (2005).
Miller, K. M., Rog, O. & Cooper, J. P. Semi-conservative DNA replication through telomeres requires Taz1. Nature 440, 824–828 (2006).
Chang, S. et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 36, 877–882 (2004).
Laud, P. R. et al. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 19, 2560–2570 (2005).
Crabbe, L., Jauch, A., Naeger, C. M., Holtgreve-Grez, H. & Karlseder, J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl Acad. Sci. USA 104, 2205–2210 (2007).
Mason, P. J. Stem cells, telomerase and dyskeratosis congenita. Bioessays 25, 126–133 (2003).
Mason, P. J., Wilson, D. B. & Bessler, M. Dyskeratosis congenita — a disease of dysfunctional telomere maintenance. Curr. Mol. Med. 5, 159–170 (2005).
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Vulliamy, T. et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413, 432–435 (2001).
Vulliamy, T. J. et al. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol. Dis. 34, 257–263 (2005).
Savage, S. A. et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 82, 501–509 (2008).
Walne, A. J., Vulliamy, T., Beswick, R., Kirwan, M. & Dokal, I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood 112, 3594–3600 (2008).
Hockemeyer, D., Palm, W., Wang, R. C., Couto, S. S. & de Lange, T. Engineered telomere degradation models dyskeratosis congenita. Genes Dev. 22, 1773–1785 (2008).
Alder, J. K. et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA 105, 13051–13056 (2008).
Armanios, M. Y. et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 (2007).
Greider, C. W. & Blackburn, E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898 (1987). This manuscript is the first one to suggest that telomerase is required for elongation of chromosome ends in eukaryotes.
Cohen, S. B. et al. Protein composition of catalytically active human telomerase from immortal cells. Science 315, 1850–1853 (2007).
Hemann, M. T., Strong, M. A., Hao, L. Y. & Greider, C. W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107, 67–77 (2001).
Marcand, S., Brevet, V. & Gilson, E. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 18, 3509–3519 (1999).
Zhao, Y. et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 138, 463–475 (2009).
Blasco, M. A. Telomere length, stem cells and aging. Nature Chem. Biol. 3, 640–649 (2007).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Masutomi, K. et al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl Acad. Sci. USA 102, 8222–8227 (2005).
Sarin, K. Y. et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048–1052 (2005).
Bryan, T. M., Englezou, A., Gupta, J., Bacchetti, S. & Reddel, R. R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14, 4240–4248 (1995).
Yeager, T. R. et al. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 59, 4175–4179 (1999).
Luger, K. Structure and dynamic behavior of nucleosomes. Curr. Opin. Genet. Dev. 13, 127–135 (2003).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Caslini, C., Connelly, J. A., Serna, A., Broccoli, D. & Hess, J. L. MLL associates with telomeres and regulates telomeric repeat-containing RNA transcription. Mol. Cell Biol. 29, 4519–4526 (2009).
Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462, 315–322 (2009).
Probst, A. V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nature Rev. Mol. Cell Biol. 10, 192–206 (2009).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
Marion, R. M. et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell 4, 141–154 (2009).
Acknowledgements
R.O.S. is supported by the George E. Hewitt Foundation for Medical Research and J.K. acknowledges support by the National Institutes of Health (RO1 GM06525 and RO1 AG025837). We thank A. Cesare, D. Lackner, C. Naeger and L. Oganesian for images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
Autosomal dominant dyskeratosis congenita
X-linked recessive dyskeratosis congenita
FURTHER INFORMATION
Glossary
- G-quadruplex
-
A higher-order DNA structure consisting of G quartets in which guanosine residues are donors and acceptors in a G–G base pair. This structure is an obstacle to the moving replication fork.
- Fragile site
-
A site in a chromosome at which breaks frequently occur.
- Displacement loop
-
A single-stranded DNA loop, resulting from the invasion and pairing of a DNA end into homologous double-stranded sequences.
- Replicative senescence
-
A permanently differentiated state that cells enter when their telomeres become critically short or a threshold of DNA damage is exceeded.
- Homologous recombination
-
A repair pathway in which homologous sequences align and genetic information is copied from one DNA strand to the other.
- Werner syndrome
-
An inherited genetic disease that is characterized by premature-ageing symptoms and the early onset of cancer.
- Sister telomere loss
-
The loss of telomeric sequences from a single sister chromatid while the other telomere stays intact.
- Sister chromatid exchange
-
The HR-based exchange of DNA strands between sister chromatids.
- Nijmegen breakage syndrome
-
A rare syndrome characterized by chromosomal instability that is a result of mutations in the Nijmegen breakage syndrome 1 (NBS1; also known as NBN) gene.
- Ataxia telangiectasia
-
A rare, inherited disease, characterized by neurodegeneration, cancer susceptibility and radiation sensitivity, that is caused by mutations in the ATM gene.
- Non-reciprocal translocation
-
The transfer of genetic information from one non-homologous chromosome to another.
Rights and permissions
About this article
Cite this article
O'Sullivan, R., Karlseder, J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11, 171–181 (2010). https://doi.org/10.1038/nrm2848
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2848
This article is cited by
-
Adaptive and Maladaptive Clonal Hematopoiesis in Telomere Biology Disorders
Current Hematologic Malignancy Reports (2024)
-
The crosstalk between telomeres and DNA repair mechanisms: an overview to mammalian somatic cells, germ cells, and preimplantation embryos
Journal of Assisted Reproduction and Genetics (2024)
-
Chromosome territory reorganization through artificial chromosome fusion is compatible with cell fate determination and mouse development
Cell Discovery (2023)
-
Crosstalk between G-quadruplex and ROS
Cell Death & Disease (2023)
-
Aerobic exercise and telomere length in patients with systolic heart failure: protocol study for a randomized controlled trial
Trials (2022)