Key Points
-
Borrelia burgdorferi, the spirochaete that causes Lyme disease, is a fastidious, slow-growing bacterium. This spirochaete has a complex genome composed of multiple linear and circular plasmids, in addition to a linear chromosome.
-
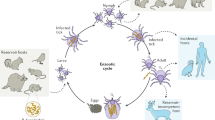
The B. burgdorferi natural life cycle alternates between ticks and small mammals and can be reproduced in the laboratory. Ticks can also be directly infected with B. burgdorferi by several methods.
-
Although genetic studies have only been undertaken in the past ten years, a number of genetic tools have been developed. Multiple selectable markers have been used for gene inactivation by allelic exchange. Shuttle vectors derived from endogenous and broad host-range plasmids have been constructed. A transposon mutagenesis system has been used to inactivate a number of genes.
-
Limitations on genetic studies of B. burgdorferi remain. Transformation frequencies are very low, especially in infectious strains, in part because of plasmid-encoded restriction enzymes. Plasmids are unstable during in vitro growth, so care must be taken to ensure isogenicity of mutant and wild-type strains. Currently, the bacteria are only grown in complex medium, so many nutritional screens and selections are not possible.
-
Despite these limitations, genes affecting a number of functions, including infection of mice or ticks, have been inactivated. Also, a gene that is essential for survival in all conditions has been identified.
Abstract
Lyme disease is the most commonly reported vector-borne disease in North America and Europe, yet we know little about which components of the causative agent, Borrelia burgdorferi, are critical for infection or virulence. Molecular genetics has provided a powerful means by which to address these topics in other bacterial pathogens. Certain features of B. burgdorferi have hampered the development of an effective system of genetic analysis, but basic tools are now available and their application has begun to provide information about the identities and roles of key bacterial components in both the tick vector and the mammalian host. Increased genetic analysis of B. burgdorferi should advance our understanding of the infectious cycle and the pathogenesis of Lyme disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Burgdorfer, W. et al. Lyme disease — a tick-borne spirochetosis? Science 216, 1317–1319 (1982). Historically significant, the first paper to identify the bacterial agent of Lyme disease.
Benach, J. L. et al. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308, 740–742 (1983).
Steere, A. C. et al. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308, 733–740 (1983).
Paster, B. J., Stackebrandt, E., Hespell, R. B., Hahn, C. M. & Woese, C. R. The phylogeny of the spirochetes. Syst. Appl. Microbiol. 5, 337–351 (1984).
Woese, C. R. Bacterial evolution. Microbiol. Rev. 51, 221–271 (1987).
Sohaskey, C. D., Arnold, C. & Barbour, A. G. Analysis of promoters in Borrelia burgdorferi by use of a transiently expressed reporter gene. J. Bacteriol. 179, 8637–8642 (1997).
Stevenson, B., Bono, J. L., Elias, A., Tilly, K. & Rosa, P. Transformation of the Lyme disease spirochete Borrelia burgdorferi with heterologous DNA. J. Bacteriol. 180, 4850–4855 (1998).
Bono, J. L. et al. Efficient targeted mutagenesis in Borrelia burgdorferi . J. Bacteriol. 182, 2445–2452 (2000).
Stewart, P. E., Thalken, R., Bono, J. L. & Rosa, P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi . Mol. Microbiol. 39, 714–721 (2001).
Johnson, R. C. The spirochetes. Annu. Rev. Microbiol. 31, 89–106 (1977).
Klaviter, E. C. & Johnson, R. C. Isolation of the outer envelope, chemical components, and ultrastructure of Borrelia hermsii grown in vitro . Acta Trop. 36, 123–131 (1979).
Hovind-Hougen, K. Ultrastructure of spirochetes isolated from Ixodes ricinus and Ixodes dammini . Yale J. Biol. Med. 57, 543–548 (1984).
Barbour, A. G. & Hayes, S. F. Biology of Borrelia species. Microbiol. Rev. 50, 381–400 (1986).
Coleman, J. L., Benach, J. L., Beck, G. & Habicht, G. S. Isolation of the outer envelope from Borrelia burgdorferi . Zbl. Bakt. Hyg. A 263, 123–126 (1986).
Takayama, K., Rothenberg, R. J. & Barbour, A. G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi . Infect. Immun. 55, 2311–2313 (1987).
Fraser, C. M. et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi . Nature 390, 580–586 (1997).
Samuels, D. S., Mach, K. E. & Garon, C. F. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB . J. Bacteriol. 176, 6045–6049 (1994). The first report of genetic transformation in B. burgdorferi , and therefore a historically significant paper that laid the foundation for all subsequent genetic manipulations of this spirochaete.
Tilly, K., Elias, A. F., Bono, J. L., Stewart, P. & Rosa, P. DNA exchange and insertional inactivation in spirochetes. J. Mol. Microbiol. Biotechnol. 2, 433–442 (2000).
Casjens, S. et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi . Mol. Microbiol. 35, 490–516 (2000). An encyclopaedic report, this paper describes the evolutionary relationships and nucleotide sequences of the entire plasmid complement of type strain B31.
Xu, Y., Kodner, C., Coleman, L. & Johnson, R. C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect. Immun. 64, 3870–3876 (1996).
Schwan, T. G., Burgdorfer, W. & Garon, C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56, 1831–1836 (1988).
Purser, J. E. & Norris, S. J. Correlation between plasmid content and infectivity in Borrelia burgdorferi . Proc. Natl Acad. Sci. USA 97, 13865–13870 (2000).
Labandeira-Rey, M. & Skare, J. T. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69, 446–455 (2001).
Zhang, J. -R., Hardham, J. M., Barbour, A. G. & Norris, S. J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89, 275–285 (1997). The first description of the genetic mechanism of antigenic variation in B. burgdorferi.
Elias, A. F. et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70, 2139–2150 (2002). This paper describes the inherent difficulties of genetically manipulating nonclonal strains. Elias and colleagues isolated and characterized a clonal B. burgdorferi strain that was amenable to site-directed mutagenesis.
Grimm, D., Elias, A. F., Tilly, K. & Rosa, P. A. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 71, 3138–3145 (2003).
Labandeira-Rey, M., Seshu, J. & Skare, J. T. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71, 4608–4613 (2003).
Purser, J. E., Lawrenz, M. B., Caimano, M. J., Radolf, J. D. & Norris, S. J. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48, 753–764 (2003). Describes the identification of a B. burgdorferi plasmid gene that fulfils an essential physiological function and is required for mammalian infectivity. This paper is the first to fulfil molecular Koch's postulates for a Borrelia gene.
Rosa, P. A., Schwan, T. & Hogan, D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi . Mol. Microbiol. 6, 3031–3040 (1992).
Zhang, J. R. & Norris, S. J. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi . Infect. Immun. 66, 3869–3697 (1998).
Piesman, J., Mather, T. M., Sinsky, R. J. & Spielman, A. Duration of tick attachment and Borrelia burgdorferi transmission. J. Clin. Microbiol. 25, 557–558 (1987).
Piesman, J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi . J. Med. Entomol. 30, 199–203 (1993).
Nakayama, Y. & Spielman, A. Ingestion of Lyme disease spirochetes by ticks feeding on infected hosts. J. Infect. Dis. 160, 166–167 (1989).
Gern, L., Zhu, Z. & Aeschlimann, A. Development of Borrelia burgdorferi in Ixodes ricinus females during blood feeding. Ann. Parasitol. Hum. Comp. 65, 89–93 (1990).
Barthold, S. W., Beck, D. S., Hansen, G. M., Terwilliger, G. A. & Moody, K. D. Experimental Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162, 133–138 (1990).
Salyers, A. A. & Whitt, D. D. Bacterial Pathogenesis: a Molecular Approach (ASM Press, Washington DC, 2001).
Snyder, L. & Champness, W. Molecular Genetics of Bacteria (ASM Press, Washington DC, 2002).
Bosler, E. M. et al. Natural distribution of the Ixodes dammini spirochete. Science 220, 321–322 (1982).
Lane, R. S., Piesman, J. & Burgdorfer, W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36, 587–609 (1991).
Magnarelli, L. A. & Anderson, J. F. Ticks and biting insects infected with the etiologic agent of Lyme disease, Borrelia burgdorferi . J. Clin. Microbiol. 26, 1482–1486 (1988).
Matuschka, F. -R., Fischer, P., Heiler, M., Richter, D. & Spielman, A. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J. Infect. Dis. 165, 479–483 (1992).
Olsen, B., Jaenson, T. G. T., Noppa, L., Bunikis, J. & Bergström, S. A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature 362, 340–342 (1993).
Burgdorfer, W., Hayes, S. F. & Benach, J. L. in Lyme Disease and Related Disorders (eds Benach, J. L. & Bosler, E. M.) 172–179 (New York Academy of Sciences, New York, 1988).
Barthold, S. W., Persing, D. H., Armstrong, A. L. & Peeples, R. A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139, 263–273 (1991).
Philipp, M. T. & Johnson, B. J. B. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 2, 431–437 (1994).
Donahue, J. G., Piesman, J. & Spielman, A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 36, 92–96 (1987).
Burkot, T. R., Happ, C. M., Dolan, M. C. & Maupin, G. O. Infection of Ixodes scapularis (Acari: Ixodidae) with Borrelia burgdorferi using a new artificial feeding technique. J. Med. Entomol. 38, 167–171 (2001).
Broadwater, A. H., Sonenshine, D. E., Hynes, W. L., Ceraul, S. & de Silva, A. M. Glass capillary tube feeding: a method for infecting nymphal Ixodes scapularis (Acari: Ixodidae) with the Lyme disease spirochete Borrelia burgdorferi . J. Med. Entomol. 39, 285–292 (2002).
Policastro, P. F. & Schwan, T. G. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi . J. Med. Entomol. 40, 364–370 (2003).
Narasimhan, S. et al. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc. Natl Acad. Sci. USA 101, 1141–1146 (2004). An interesting study that demonstrates the potential uses of RNAi to study vector–parasite interactions.
Piesman, J., Oliver, J. R. & Sinsky, R. J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 42, 352–357 (1990).
Piesman, J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J. Infect. Dis. 167, 1082–1085 (1993).
Piesman, J. Dispersal of the Lyme disease spirochete Borrelia burgdorferi to salivary glands of feeding nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 32, 519–521 (1995).
Ribeiro, J. M. C., Mather, T. N., Piesman, J. & Spielman, A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 24, 201–205 (1987).
Zung, J. L. et al. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini . Can. J. Zool. 67, 1737–1748 (1989).
Schwan, T. G., Piesman, J., Golde, W. T., Dolan, M. C. & Rosa, P. A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl Acad. Sci. USA 92, 2909–2913 (1995). A historically significant paper identifying temperature as a key environmental signal that B. burgdorferi senses and responds to by remodelling its outer surface.
Carroll, J. A., Garon, C. F. & Schwan, T. G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi . Infect. Immun. 67, 3181–3187 (1999).
Indest, K. J. et al. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro . Infect. Immun. 65, 1165–1171 (1997).
Yang, X. et al. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi . Mol. Microbiol. 37, 1470–1479 (2000).
Tokarz, R., Anderton, J. M., Katona, L. I. & Benach, J. L. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72, 5419–5432 (2004).
Suk, K. et al. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl Acad. Sci. USA 92, 4269–4273 (1995).
Fikrig, E. et al. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31, 281–290 (1999).
Liang, F. T., Nelson, F. K. & Fikrig, E. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196, 275–280 (2002).
Akins, D. K., Bourell, K. W., Caimano, M. J., Norgard, M. V. & Radolf, J. D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101, 2240–2250 (1998).
Brooks, C. S., Hefty, P. S., Jolliff, S. E. & Akins, D. R. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71, 3371–3383 (2003).
Revel, A. T., Talaat, A. M. & Norgard, M. V. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl Acad. Sci. USA 99, 1562–1567 (2002).
Jonsson, M., Elmros, T. & Bergstrom, S. Subcutaneous implanted chambers in different mouse strains as an animal model to study genetic stability during infection with Lyme disease Borrelia . Microb. Pathog. 18, 109–114 (1995).
Crother, C. R. et al. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71, 3419–3428 (2003).
Crother, T. R. et al. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 72, 5063–5072 (2004).
Rosa, P. et al. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi . J. Bacteriol. 178, 5946–5953 (1996).
Elias, A. F. et al. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi . J. Mol. Microbiol. Biotechnol. 6, 29–40 (2003).
Sartakova, M., Dobrikova, E. & Cabello, F. C. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi . Proc. Natl Acad. Sci. USA 97, 4850–4855 (2000).
Sartakova, M. L. et al. Novel antibiotic-resistance markers in pGK12-derived vectors for Borrelia burgdorferi . Gene 303, 131–137 (2003).
Frank, K. L., Bundle, S. F., Kresge, M. E., Eggers, C. H. & Samuels, D. S. aadA confers streptomycin resistance in Borrelia burgdorferi . J. Bacteriol. 185, 6723–6727 (2003).
Eggers, C. H. et al. Transduction by φBB-1, a bacteriophage of Borrelia burgdorferi . J. Bacteriol. 183, 4771–4778 (2001).
Stewart, P. E., Chaconas, G. & Rosa, P. Conservation of plasmid maintenance functions between linear and circular plasmids in Borrelia burgdorferi . J. Bacteriol. 185, 3202–3209 (2003).
Eggers, C. H. et al. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43, 281–295 (2002).
Byram, R., Stewart, P. E. & Rosa, P. A. The essential nature of the ubiquitous 26-kb circular replicon of Borrelia burgdorferi . J. Bacteriol. 186, 3561–3569 (2004).
Carroll, J., Stewart, P., Rosa, P. & Garon, C. An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi . Microbiology 149, 1819–1828 (2003).
Alverson, J., Bundle, S. F., Sohaskey, C. D., Lybecker, M. C. & Samuels, D. S. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi . Mol. Microbiol. 48, 1665–1677 (2003).
Stewart, P. E., Hoff, J., Fischer, E., Krum, J. G. & Rosa, P. A. Genome-wide transposon mutagenesis of Borrelia burgdorferi for identification of phenotypic mutants. Appl. Environ. Microbiol. 70, 5973–5979 (2004).
Lawrenz, M. B., Kawabata, H., Purser, J. E. & Norris, S. J. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious Borrelia . Infect. Immun. 70, 4851–4858 (2002).
Tilly, K., Grimm, D., Bueschel, D. M., Krum, J. G. & Rosa, P. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis. 4, 159–186 (2004).
Grimm, D. et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl Acad. Sci. USA 101, 3142–3147 (2004). The first paper to disrupt and complement a B. burgdorferi gene and monitor the modified strains throughout the complete infectious cycle (mouse to tick to mouse).
Grimm, D. et al. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of B. burgdorferi throughout the infectious cycle. Infect. Immun. 72, 5938–5946 (2004).
Kelly, R. Cultivation of Borrelia hermsii . Science 173, 443–444 (1971).
Barbour, A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57, 521–525 (1984).
Hubner, A. et al. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN–RpoS regulatory pathway. Proc. Natl Acad. Sci. USA 98, 12724–12729 (2001). Global regulatory networks are poorly defined in B. burgdorferi , and this paper begins to define a key regulatory system and the affected genes.
Kimsey, R. B. & Spielman, A. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 162, 1205–1208 (1990).
Charon, N. W. & Goldstein, S. F. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu. Rev. Genet. 36, 47–73 (2002).
Motaleb, M. A. et al. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl Acad. Sci. USA 97, 10899–10904 (2000). This paper demonstrates that the spirochaete morphology of B. burgdorferi is conferred by the periplasmic flagella, not the peptidoglycan layer, which is responsible for conferring morphology in most other bacteria.
Sartakova, M. L. et al. Complementation of a nonmotile flaB mutant of Borrelia burgdorferi by chromosomal integration of a plasmid containing a wild-type flaB allele. J. Bacteriol. 183, 6558–6564 (2001).
Motaleb, M. A., Sal, M. S. & Charon, N. W. The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. J. Bacteriol. 186, 3703–3711 (2004).
Ge, Y., Old, I. G., Girons, I. S. & Charon, N. W. The flgK motility operon of Borrelia burgdorferi is initiated by a sigma 70-like promoter. Microbiology 143, 1861–1690 (1997).
Ge, Y. & Charon, N. W. Identification of a large motility operon in Borrelia burgdorferi by semi-random PCR chromosome walking. Gene 189, 195–201 (1997).
Ge, Y., Old, I. G., Saint Girons, I. & Charon, N. W. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus sigma70 promoter. J. Bacteriol. 179, 2289–2299 (1997).
Ge, Y., Li, C., Corum, L., Slaughter, C. A. & Charon, N. W. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi . J. Bacteriol. 180, 2418–2425 (1998).
Li, C. et al. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc. Natl Acad. Sci. USA 99, 6169–6174 (2002).
Chaconas, G., Stewart, P. E., Tilly, K., Bono, J. L. & Rosa, P. Telomere resolution in the Lyme disease spirochete. EMBO J. 20, 3229–3237 (2001).
Barbour, A. G. & Garon, C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science 237, 409–411 (1987). This paper elucidates the unusual structure of the linear DNA molecules of B. burgdorferi.
Kobryn, K. & Chaconas, G. The circle is broken: telomere resolution in linear replicons. Curr. Opin. Microbiol. 4, 558–564 (2001).
Kobryn, K. & Chaconas, G. ResT, a telomere resolvase encoded by the Lyme disease spirochete. Mol. Cell 9, 195–201 (2002). Describes the identification and characterization of the enzyme necessary for maintaining the hairpin ends of the linear DNA molecules of B. burgdorferi.
Deneke, J., Ziegelin, G., Lurz, R. & Lanka, E. The protelomerase of temperate Escherichia coli phage N15 has cleaving–joining activity. Proc. Natl Acad. Sci. USA 97, 7721–7726 (2000).
Margolis, N., Hogan, D., Tilly, K. & Rosa, P. A. Plasmid location of Borrelia purine biosynthesis gene homologs. J. Bacteriol. 176, 6427–6432 (1994).
Bono, J. L., Tilly, K., Stevenson, B., Hogan, D. & Rosa, P. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144, 1033–1044 (1998).
Sadziene, A., Wilske, B., Ferdows, M. S. & Barbour, A. G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect. Immun. 61, 2192–2195 (1993).
Marconi, R. T., Samuels, D. S. & Garon, C. F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J. Bacteriol. 175, 926–932 (1993).
Ohnishi, J., Piesman, J. & de Silva, A. M. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl Acad. Sci. USA 98, 670–675 (2001).
Barbour, A. G. Linear DNA of Borrelia species and antigenic variation. Trends Microbiol. 1, 236–239 (1993).
Tilly, K. et al. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi . J. Bacteriol. 183, 5544–5553 (2001).
Pal, U. et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113, 220–230 (2004).
Stevenson, B. & Babb, K. LuxS-mediated quorum sensing in Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 70, 4099–4105 (2002).
Hubner, A., Revel, A. T., Nolen, D. M., Hagman, K. E. & Norgard, M. V. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect. Immun. 71, 2892–2896 (2003).
Blevins, J. S. et al. The luxS gene is not required for Borrelia burgdorferi tick colonization, transmission to a mammalian host or induction of disease. Infect. Immun. 72, 4864–4867 (2004).
de Silva, A. M., Telford, S. R., Brunet, L. R., Barthold, S. W. & Fikrig, E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183, 271–275 (1996).
Schwan, T. G. & Piesman, J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 39, 382–388 (2000).
Yang, X. F., Alani, S. M. & Norgard, M. V. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi . Proc. Natl Acad. Sci. USA 100, 11001–11006 (2003).
Seshu, J., Boylan, J. A., Gherardini, F. C. & Skare, J. T. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi . Infect. Immun. 72, 1580–1586 (2004).
Yang, X. F., Pal, U., Alani, S. M., Fikrig, E. & Norgard, M. V. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199, 641–648 (2004).
Liang, F. T., Jacobs, M. B., Bowers, L. C. & Philipp, M. T. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195, 415–422 (2002).
Eicken, C. et al. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi . J. Biol. Chem. 276, 10010–10015 (2001).
Kumaran, D. et al. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi . EMBO J. 20, 971–978 (2001).
Howe, T. R., Mayer, L. W. & Barbour, A. G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science 227, 645–646 (1985).
Barbour, A. G. & Garon, C. F. in Lyme Disease and Related Disorders (eds Benach, J. L. & Bosler, E. M.) 144–153 (New York Academy of Sciences, New York, 1988).
Sadziene, A., Thomas, D. D. & Barbour, A. G. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect. Immun. 63, 1573–1580 (1995).
Pal, U. et al. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Invest. 106, 561–569 (2000).
Li, H., Dunn, J. J., Luft, B. J. & Lawson, C. L. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc. Natl Acad. Sci. USA 94, 3584–3589 (1997).
Eicken, C. et al. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi . J. Biol. Chem. 277, 21691–21696 (2002).
Ojaimi, C. et al. Profiling temperature-induced changes in Borrelia burgdorferi gene expression using whole genome arrays. Infect. Immun. 71, 1869–1705 (2003).
Narasimhan, S. et al. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J. Bacteriol. 184, 3122–3125 (2002).
Narasimhan, S. et al. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc. Natl Acad. Sci. USA 100, 15953–15958 (2003).
Gray, J. S., Kahl, O., Lane, R. S. & Stanek, G. (eds). Lyme Borreliosis: Biology, Epidemiology and Control (CABI Publishing, UK, 2002).
Baranton, G. et al. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42, 378–383 (1992).
Kawabata, H., Masuzawa, T. & Yanagihara, Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol. Immunol. 37, 843–848 (1993).
Steere, A. C. Lyme disease. N. Engl. J. Med. 345, 115–125 (2001).
van Dam, A. P. et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17, 708–717 (1993).
Paster, B. J. & Dewhirst, F. E. Phylogenetic foundation of spirochetes. J. Mol. Microbiol. Biotechnol. 2, 341–344 (2000).
Picardeau, M., Brenot, A. & Saint Girons, I. First evidence for gene replacement in Leptospira spp., inactivation of L. biflexa flaB results in non-motile mutants deficient in endoflagella. Mol. Microbiol. 40, 189–199 (2001).
ter Huurne, A. A. H. M. et al. Inactivation of a Serpula (Treponema) hyodysenteriae hemolysin gene by homologous recombination: importance of this hemolysin in pathogenesis of S. hyodysenteriae in mice. FEMS Microbiol. Lett. 92, 109–114 (1992).
Rosey, E. L., Kennedy, M. J., Petrella, D. K., Ulrich, R. G. & Yancey, R. J. Jr. Inactivation of Serpulina hyodysenteriae flaA1 and flaB1 periplasmic flagellar genes by electroporation-mediated allelic exchange. J. Bacteriol. 177, 5959–5970 (1995).
Stanton, T. B., Rosey, E. L., Kennedy, M. J., Jensen, N. S. & Bosworth, B. T. Isolation, oxygen sensitivity, and virulence of NADH oxidase mutants of the anaerobic spirochete Brachyspira (Serpulina) hyodysenteriae, etiologic agent of swine dysentery. Appl. Environ. Microbiol. 65, 5028–5034 (1999).
Li, H. & Kuramitsu, H. K. Development of a gene transfer system in Treponema denticola by electroporation. Oral Microbiol. Immunol. 11, 161–165 (1996).
Saint Girons, I., Margarita, D., Amouriaux, P. & Baranton, G. First isolation of bacteriophages for a spirochaete: potential genetic tools for Leptospira . Res. Microbiol. 141, 1131–1138 (1990).
Fraser, C. M. et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281, 375–388 (1998).
Nascimento, A. L. et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186, 2164–2172 (2004).
Seshadri, R. et al. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc. Natl Acad. Sci. USA 101, 5646–5651 (2004).
Ren, S. X. et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422, 888–893 (2003).
Fracek, S. P. Jr & Stolz, J. F. Spirochaeta bajacaliforniensis sp. n. from a microbial mat community at Laguna Figueroa, Baja California Norte, Mexico. Arch. Microbiol. 142, 317–325 (1985).
Qiu, W. -G. et al. Genetic exchange and plasmid transfers in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. Proc. Natl Acad. Sci. USA 101, 14150–14155 (2004).
Miller, J. C. et al. A second allele of eppA in Borrelia burgdorferi strain B31 is located on the previously undetected circular plasmid cp9-2. J. Bacteriol. 182, 6254–6258 (2000).
Palmer, N., Fraser, C. & Casjens, S. Distribution of twelve linear extrachromosomal DNAs in natural isolates of Lyme disease spirochetes. J. Bacteriol. 182, 2476–2480 (2000).
Iyer, R. et al. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 71, 3699–3706 (2003).
Stevenson, B. & Miller, J. C. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol. 57, 309–324 (2003).
Dykhuizen, D. E. et al. Borrelia burgdorferi is clonal: implications for taxonomy and vaccine development. Proc. Natl Acad. Sci. USA 90, 10163–10167 (1993).
Stevenson, B., Tilly, K. & Rosa, P. A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J. Bacteriol. 178, 3508–3516 (1996).
Caimano, M. J. et al. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect. Immun. 86, 1574–1586 (2000).
Eggers, C. H. & Samuels, D. S. Molecular evidence for a new bacteriophage of Borrelia burgdorferi . J. Bacteriol. 181, 7308–7313 (1999).
Barbour, A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J. Clin. Microbiol. 26, 475–478 (1988).
Knight, S. W., Kimmel, B. J., Eggers, C. H. & Samuels, D. S. Disruption of the Borrelia burgdorferi gac gene, encoding the naturally synthesized GyrA C-terminal domain. J. Bacteriol. 182, 2048–2051 (2000).
Elias, A. F. et al. Altered stationary phase response in a Borrelia burgdorferi rpoS mutant. J. Bacteriol. 182, 2909–2918 (2000).
Ostberg, Y., Pinne, M., Benz, R., Rosa, P. & Bergstrom, S. Elimination of channel-forming activity by insertional inactivation of the p13 gene in Borrelia burgdorferi . J. Bacteriol. 184, 8611–8619 (2002).
Coburn, J. & Cugini, C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin αvβ3. Proc. Natl Acad. Sci. USA 100, 7301–7306 (2003).
Ostberg, Y. et al. Pleiotropic effects of inactivating a carboxy-terminal protease, CtpA, in Borrelia burgdorferi . J. Bacteriol. 186, 2074–2084 (2004).
Tilly, K. et al. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol. Microbiol. 25, 361–373 (1997).
Tilly, K., Lubke, L. & Rosa, P. Characterization of circular plasmid dimers in Borrelia burgdorferi . J. Bacteriol. 180, 5676–5861 (1998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez
Infectious Disease Information
SwissProt
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Rosa, P., Tilly, K. & Stewart, P. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol 3, 129–143 (2005). https://doi.org/10.1038/nrmicro1086
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1086
This article is cited by
-
Prevention of tick-borne diseases: challenge to recent medicine
Biologia (2022)
-
Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identification and survey of tick-borne encephalitis virus in ticks from north-eastern Germany
Parasites & Vectors (2020)
-
Design of a broadly reactive Lyme disease vaccine
npj Vaccines (2020)