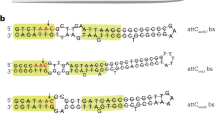
Key Points
-
Integrons are assembly platforms that incorporate exogenous open reading frames through site-specific recombination and convert them to functional genes by ensuring their correct expression.
-
Although integrons were discovered through their involvement in the development of multiple antibiotic resistance in Gram-negative pathogens when carried in transposons, their role in genome evolution has been extended with the discovery of other, often larger, integron structures as genuine components of the genomes of many γ-proteobacterial species.
-
This Review discusses the structural differences among the different types of integron — those carried in mobile DNA elements and the chromosomal superintegrons — as well as their evolutionary history and phylogenetic relationships.
-
The different functions encoded by the integron gene cassettes are reviewed, with emphasis on those from superintegrons and other chromosomal integrons from environmental bacteria.
-
The dynamics of the intraspecies and interspecies variation of the large cassette arrays of superintegrons, and their role in the increase in antibiotic resistance, are discussed.
-
Finally, the specific recombination reactions occurring in these elements are reviewed, and a novel model involving a single-stranded substrate for recombination for cassette insertion and deletion is proposed.
Abstract
Integrons are assembly platforms — DNA elements that acquire open reading frames embedded in exogenous gene cassettes and convert them to functional genes by ensuring their correct expression. They were first identified by virtue of their important role in the spread of antibiotic-resistance genes. More recently, our understanding of their importance in bacterial genome evolution has broadened with the discovery of larger integron structures, termed superintegrons. These DNA elements contain hundreds of accessory genes and constitute a significant fraction of the genomes of many bacterial species. Here, the basic biology of integrons and superintegrons, their evolutionary history and the evidence for the existence of a novel recombination pathway is reviewed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mitsuhashi, S., Harada, K., Hashimoto, H. & Egawa, R. On the drug-resistance of enteric bacteria. Jpn. J. Exp. Med. 31, 47–52 (1961).
Stokes, H. W. & Hall, R. M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3, 1669–1683 (1989). This is the first description of integrons.
Liebert, C. A., Hall, R. M. & Summers, A. O. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63, 507–522 (1999). A comprehensive review on Tn 21 and its intricate evolution and assembly.
Hall, R. M. & Collis, C. M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15, 593–600 (1995).
Collis, C. M., Grammaticopoulos, G., Briton, J., Stokes, H. W. & Hall, R. M. Site-specific insertion of gene cassettes into integrons. Mol. Microbiol. 9, 41–52 (1993).
Recchia, G. D. & Hall, R. M. Gene cassettes: a new class of mobile element. Microbiology 141, 3015–3027 (1995).
Recchia, G. D. & Sherratt, D. J. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 162–176 (ASM Press, Washington DC, 2002).
Hall, R. M. Mobile gene cassettes and integrons: moving antibiotic resistance genes in Gram-negative bacteria. Ciba Found. Symp. 207, 192–202 (1997).
Radstrom, P. et al. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J. Bacteriol. 176, 3257–3268 (1994).
Brown, H. J., Stokes, H. W. & Hall, R. M. The integrons In0, In2, and In5 are defective transposon derivatives. J. Bacteriol. 178, 4429–4437 (1996).
Sundstrom, L., Roy, P. H. & Skold, O. Site-specific insertion of three structural gene cassettes in transposon Tn7. J. Bacteriol. 173, 3025–3028 (1991).
Collis, C. M., Kim, M. J., Partridge, S. R., Stokes, H. W. & Hall, R. M. Characterization of the class 3 integron and the site-specific recombination system it determines. J. Bacteriol. 184, 3017–3026 (2002).
Shibata, N. et al. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by Gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 41, 5407–5413 (2003).
Correia, M. et al. Molecular characterization of a new class 3 integron in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 47, 2838–2843 (2003).
Arakawa, Y. et al. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP . Antimicrob. Agents Chemother. 39, 1612–1615 (1995).
Hochhut, B. et al. Molecular analysis of antibiotic resistance gene clusters in Vibrio cholerae O139 and O1 SXT constins. Antimicrob. Agents Chemother. 45, 2991–3000 (2001).
Rowe-Magnus, D. A. & Mazel, D. The role of integrons in antibiotic resistance gene capture. Int. J. Med. Microbiol. 292, 115–125 (2002).
Fluit, A. C. & Schmitz, F. J. Resistance integrons and super-integrons. Clin. Microbiol. Infect. 10, 272–288 (2004).
Biskri, L. & Mazel, D. Erythromycin esterase gene ere(A) is located in a functional gene cassette in an unusual class 2 integron. Antimicrob. Agents Chemother. 47, 3326–3331 (2003).
Ramirez, M. S., Vargas, L. J., Cagnoni, V., Tokumoto, M. & Centron, D. Class 2 integron with a novel cassette array in a Burkholderia cenocepacia isolate. Antimicrob. Agents Chemother. 49, 4418–4420 (2005).
Hansson, K., Sundstrom, L., Pelletier, A. & Roy, P. H. IntI2 integron integrase in Tn7. J. Bacteriol. 184, 1712–1721 (2002).
Collis, C. M., Recchia, G. D., Kim, M. J., Stokes, H. W. & Hall, R. M. Efficiency of recombination reactions catalyzed by class 1 integron integrase IntI1. J. Bacteriol. 183, 2535–2542 (2001). Presents a comparison of the efficiency of the different recombination reactions carried out by the class 1 integron integrase.
Nordmann, P. & Poirel, L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 56, 463–469 (2005).
Doublet, B., Weill, F. X., Fabre, L., Chaslus-Dancla, E. & Cloeckaert, A. Variant Salmonella genomic island 1 antibiotic resistance gene cluster containing a novel 3′-N-aminoglycoside acetyltransferase gene cassette, aac(3)-Id, in Salmonella enterica serovar Newport. Antimicrob. Agents Chemother. 48, 3806–3812 (2004).
Naas, T., Mikami, Y., Imai, T., Poirel, L. & Nordmann, P. Characterization of In53, a class 1 plasmid- and composite transposon-located integron of Escherichia coli which carries an unusual array of gene cassettes. J. Bacteriol. 183, 235–249 (2001).
Nesvera, J., Hochmannova, J. & Patek, M. An integron of class 1 is present on the plasmid pCG4 from Gram-positive bacterium Corynebacterium glutamicum. FEMS Microbiol. Lett. 169, 391–395 (1998).
Tauch, A., Gotker, S., Puhler, A., Kalinowski, J. & Thierbach, G. The 27.8-kb R-plasmid pTET3 from Corynebacterium glutamicum encodes the aminoglycoside adenyltransferase gene cassette aadA9 and the regulated tetracycline efflux system Tet 33 flanked by active copies of the widespread insertion sequence IS6100. Plasmid 48, 117–129 (2002).
Nandi, S., Maurer, J. J., Hofacre, C. & Summers, A. O. Gram-positive bacteria are a major reservoir of class 1 antibiotic resistance integrons in poultry litter. Proc. Natl Acad. Sci. USA 101, 7118–7122 (2004). Provides evidence of the presence of integrons in various staphylococci and corynebacteria.
Barker, A., Clark, C. A. & Manning, P. A. Identification of VCR, a repeated sequence associated with a locus encoding a hemagglutinin in Vibrio cholerae O1. J. Bacteriol. 176, 5450–5458 (1994).
Mazel, D., Dychinco, B., Webb, V. A. & Davies, J. A distinctive class of integron in the Vibrio cholerae genome. Science 280, 605–608 (1998). Describes the first superintegron and shows that integrons pre-date the use of antibiotics in medicine.
Heidelberg, J. F. et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406, 477–483 (2000).
Makino, K. et al. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361, 743–749 (2003).
Chen, C. Y. et al. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13, 2577–2587 (2003). Contains a comparative analysis of the cassette contents of the V. vulnificus superintegron.
Ruby, E. G. et al. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl Acad. Sci. USA 102, 3004–3009 (2005).
Vezzi, A. et al. Life at depth: Photobacterium profundum genome sequence and expression analysis. Science 307, 1459–1461 (2005).
Vaisvila, R., Morgan, R. D., Posfai, J. & Raleigh, E. A. Discovery and distribution of super-integrons among pseudomonads. Mol. Microbiol. 42, 587–601 (2001). First description of the superintegron in pseudomonads.
Rowe-Magnus, D. A., Guerout, A. M., Biskri, L., Bouige, P. & Mazel, D. Comparative analysis of superintegrons: engineering extensive genetic diversity in the vibrionaceae. Genome Res. 13, 428–442 (2003). First interspecies comparative analysis of the contents of superintegron cassettes.
Rowe-Magnus, D. A. et al. The evolutionary history of chromosomal super-integrons provides an ancestry for multi-resistant integrons. Proc. Natl Acad. Sci. USA 98, 652–657 (2001). The first characterization of integrons in a large range of Vibrio species and xanthomonads, and their phylogenetic relationships.
Gillings, M. R., Holley, M. P., Stokes, H. W. & Holmes, A. J. Integrons in Xanthomonas: a source of species genome diversity. Proc. Natl Acad. Sci. USA 102, 4419–4424 (2005).
Drouin, F., Melancon, J. & Roy, P. H. The IntI-like tyrosine recombinase of Shewanella oneidensis is active as an integron integrase. J. Bacteriol. 184, 1811–1815 (2002).
Leon, G. & Roy, P. H. Excision and integration of cassettes by an integron integrase of Nitrosomonas europaea. J. Bacteriol. 185, 2036–2041 (2003).
Coleman, N., Tetu, S., Wilson, N. & Holmes, A. An unusual integron in Treponema denticola. Microbiology 150, 3524–3526 (2004).
Nield, B. S. et al. Recovery of new integron classes from environmental DNA. FEMS Microbiol. Lett. 195, 59–65 (2001). Shows that integrons are common in environmental bacteria.
Stokes, H. W. et al. Gene cassette PCR: sequence-independent recovery of entire genes from environmental DNA. Appl. Environ. Microbiol. 67, 5240–5246 (2001).
Holmes, A. J. et al. The gene cassette metagenome is a basic resource for bacterial genome evolution. Environ. Microbiol. 5, 383–394 (2003).
Nemergut, D. R., Martin, A. P. & Schmidt, S. K. Integron diversity in heavy-metal-contaminated mine tailings and inferences about integron evolution. Appl. Environ. Microbiol. 70, 1160–1168 (2004).
Rowe-Magnus, D. A. & Mazel, D. Integrons: natural tools for bacterial genome evolution. Curr. Opin. Microbiol. 4, 565–569 (2001).
Messier, N. & Roy, P. H. Integron integrases possess a unique additional domain necessary for activity. J. Bacteriol. 183, 6699–6706 (2001). First functional analysis of the specific domain of the integron integrase.
Nunes-Duby, S. E., Kwon, H. J., Tirumalai, R. S., Ellenberger, T. & Landy, A. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 26, 391–406 (1998).
Rowe-Magnus, D. A., Guerout, A. -M. & Mazel, D. Super-integrons. Res. Microbiol. 150, 641–651 (1999).
Nield, B. S. et al. New enzymes from environmental cassette arrays: functional attributes of a phosphotransferase and an RNA-methyltransferase. Protein Sci. 13, 1651–1659 (2004).
Wright, G. D. & Thompson, P. R. Aminoglycoside phosphotransferases: proteins, structure, and mechanism. Frontiers Biosci. 4, D9–D21 (1999).
Abbott, S. L. & Janda, J. M. Severe gastroenteritis associated with Vibrio hollisae infection: report of two cases and review. Clin. Infect. Dis. 18, 310–312 (1994).
Ogawa, A. & Takeda, T. The gene encoding the heat-stable enterotoxin of Vibrio cholerae is flanked by 123-base pair direct repeats. Microbiol. Immunol. 37, 607–616 (1993).
Barker, A. & Manning, P. A. VlpA of Vibrio cholerae O1: the first bacterial member of the α 2-microglobulin lipocalin superfamily. Microbiology 143, 1805–1813 (1997).
Smith, A. B. & Siebeling, R. J. Identification of genetic loci required for capsular expression in Vibrio vulnificus. Infect. Immun. 71, 1091–1097 (2003).
Boucher, Y. et al. Recovery and evolutionary analysis of complete integron gene cassette arrays from Vibrio. BMC Evol. Biol. 6, 3 (2006).
Pandey, D. P. & Gerdes, K. Toxin–antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33, 966–976 (2005).
Rowe-Magnus, D. A., Guerout, A. M. & Mazel, D. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol. Microbiol. 43, 1657–1669 (2002).
Yildiz, F. H., Liu, X. S., Heydorn, A. & Schoolnik, G. K. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 53, 497–515 (2004).
Clark, C. A., Purins, L., Kaewrakon, P., Focareta, T. & Manning, P. A. The Vibrio cholerae O1 chromosomal integron. Microbiology 146, 2605–2612 (2000).
Dziejman, M. et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl Acad. Sci. USA 102, 3465–3470 (2005).
Collis, C. M. & Hall, R. M. Gene cassettes from the insert region of integrons are excised as covalently closed circles. Mol. Microbiol. 6, 2875–2885 (1992).
Melano, R. et al. New carbenicillin-hydrolyzing β-lactamase (CARB-7) from Vibrio cholerae non-O1, non-O139 strains encoded by the VCR region of the V. cholerae genome. Antimicrob. Agents Chemother. 46, 2162–2168 (2002).
Petroni, A. et al. CARB-9, a carbenicillinase encoded in the VCR region of Vibrio cholerae non-O1, non-O139 belongs to a family of cassette-encoded β-lactamases. Antimicrob. Agents Chemother. 48, 4042–4046 (2004).
Lim, D. et al. Insights into the molecular basis for the carbenicillinase activity of PSE-4 β-lactamase from crystallographic and kinetic studies. Biochemistry 40, 395–402 (2001).
Tennstedt, T., Szczepanowski, R., Braun, S., Puhler, A. & Schluter, A. Occurrence of integron-associated resistance gene cassettes located on antibiotic resistance plasmids isolated from a wastewater treatment plant. FEMS Microbiol. Ecol. 45, 239–252 (2003).
Azaro, M. A. & Landy, A. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 118–148 (ASM Press, Washington DC, 2002).
Martinez, E. & de la Cruz, F. Genetic elements involved in Tn21 site-specific integration, a novel mechanism for the dissemination of antibiotic resistance genes. EMBO J. 9, 1275–1281 (1990). First functional study of the integron recombination reaction, demonstrating that recombination is controlled by the encoded IntI integrase.
Francia, M. V. & Garcia Lobo, J. M. Gene integration in the Escherichia coli chromosome mediated by Tn21 integrase (Int21). J. Bacteriol. 178, 894–898 (1996).
Francia, M. V., Avila, P., de la Cruz, F. & Garcia Lobo, J. M. A hot spot in plasmid F for site-specific recombination mediated by Tn21 integron integrase. J. Bacteriol. 179, 4419–4425 (1997).
Recchia, G. D., Stokes, H. W. & Hall, R. M. Characterisation of specific and secondary recombination sites recognised by the integron DNA integrase. Nucleic Acids Res. 22, 2071–2078 (1994).
Recchia, G. D. & Hall, R. M. Plasmid evolution by acquisition of mobile gene cassettes: plasmid pIE723 contains the aadB gene cassette precisely inserted at a secondary site in the IncQ plasmid RSF1010. Mol. Microbiol. 15, 179–187 (1995).
Hansson, K., Skold, O. & Sundstrom, L. Non-palindromic attl sites of integrons are capable of site-specific recombination with one another and with secondary targets. Mol. Microbiol. 26, 441–453 (1997).
Francia, M. V., de la Cruz, F. & Garcia Lobo, J. M. Secondary-sites for integration mediated by the Tn21 integrase. Mol. Microbiol. 10, 823–828 (1993).
Holmes, A. J. et al. Recombination activity of a distinctive integron-gene cassette system associated with Pseudomonas stutzeri populations in soil. J. Bacteriol. 185, 918–928 (2003).
Biskri, L., Bouvier, M., Guerout, A. M., Boisnard, S. & Mazel, D. Comparative study of class 1 integron and Vibrio cholerae superintegron integrase activities. J. Bacteriol. 187, 1740–1750 (2005).
Van Duyne, G. D. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 93–117 (ASM Press, Washington DC, 2002).
Gravel, A., Fournier, B. & Roy, P. H. DNA complexes obtained with the integron integrase IntI1 at the attI1 site. Nucleic Acids Res. 26, 4347–4355 (1998).
Collis, C. M., Kim, M. J., Stokes, H. W. & Hall, R. M. Binding of the purified integron DNA integrase Intl1 to integron- and cassette-associated recombination sites. Mol. Microbiol. 29, 477–490 (1998).
Stokes, H. W., O'Gorman, D. B., Recchia, G. D., Parsekhian, M. & Hall, R. M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26, 731–745 (1997). First analysis of the structural characteristics of attC sites.
Hall, R. M., Brookes, D. E. & Stokes, H. W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol. Microbiol. 5, 1941–1959 (1991).
Francia, M. V., Zabala, J. C., de la Cruz, F. & Garcia-Lobo, J. M. The IntI1 integron integrase preferentially binds single-stranded DNA of the attC site. J. Bacteriol. 181, 6844–6849 (1999). Relates the discovery of the recognition of the attC site (bottom strand) by the integrase IntI1.
Johansson, C., Kamali-Moghaddam, M. & Sundstrom, L. Integron integrase binds to bulged hairpin DNA. Nucleic Acids Res. 32, 4033–4043 (2004).
Bouvier, M., Demarre, G. & Mazel, D. Integron cassette insertion: a recombination process involving a folded single-strand substrate. EMBO J. 24, 4356–4367 (2005). In vivo demonstration of single-stranded recombination and proposal of a new recombination model involving an attC single-stranded substrate.
MacDonald, D., Demarre, G., Bouvier, M., Mazel, D. & Gopaul, D. N. Structural basis for broad DNA specificity in integron recombination. Nature 440, 1157–1162 (2006). Structural studies showing how the integrase recognizes the attC -site bottom strand and how the structural characteristics of these complexes fit the requirements of the single-stranded recombination model.
Val, M. E. et al. The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Mol. Cell 19, 559–566 (2005). An example of single-strand recombination in a different system.
Walter, A. E. et al. Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions of RNA folding. Proc. Natl Acad. Sci. USA 91, 9218–9222 (1994).
Acknowledgements
Work in the Mazel laboratory was supported by the Institut Pasteur, the Centre National de la Recherche Scientifique (CNRS), the Programme de Recherche en Microbiologie (from the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche; MENESR), the Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER) and the European Union Collective Research on Aquaculture Biofouling (CRAB) consortium and Network of Excellence EuroPathoGenomics).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Transposon
-
A mobile DNA element that can relocate within the genome of its host.
- Insertion sequence
-
(IS) A small (<2.5 kb), generally phenotypically cryptic segment of DNA that has a simple organization and is capable of insertion at multiple sites in a target DNA molecule. Examples include IS1, IS608 and IS911.
- Conjugative plasmid
-
A plasmid that can move from one cell to another during the process of conjugation.
- SXT element
-
Vibrio cholerae-derived integrating and conjugative element (also referred to as a conjugative transposon or constin).
- Compound transposon
-
A segment of DNA flanked by two similar insertion sequences, in direct or inverted orientations. Examples include Tn5 and Tn10.
- Transposase
-
The enzyme that promotes cutting of the DNA at the ends of a transposable element and joining to the DNA molecule into which the element is to be inserted.
- Transposon-mutagenesis screen
-
The use of transposons to generate (knock-out) mutations.
- Nudix hydrolases
-
Nudix hydrolases (nucleoside diphosphate linked to some other moiety X) are enzymes that hydrolyse diverse nucleoside diphosphate or triphosphate derivatives.
- Toxin–antitoxin families
-
Paired loci found in the chromosomes of almost all free-living bacteria, and many plasmids and phage genomes. They encode a toxin and its antidote, which have been shown to contribute to plasmid stability by a mechanism called post-segregational killing and are also proposed to function in bacterial programmed cell death or stress physiology.
- Minimum inhibitory concentration
-
The lowest concentration of an antibiotic that inhibits growth of the organism.
- Co-integrates
-
The structural fusion of replicons through a recombination reaction.
- Holliday junction
-
A point at which the strands of two double-stranded DNA molecules exchange partners, which occurs as an intermediate in genetic recombination.
Rights and permissions
About this article
Cite this article
Mazel, D. Integrons: agents of bacterial evolution. Nat Rev Microbiol 4, 608–620 (2006). https://doi.org/10.1038/nrmicro1462
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1462
This article is cited by
-
Prevalence, molecular characterization of integrons and its associated gene cassettes in Klebsiella pneumoniae and K. oxytoca recovered from diverse environmental matrices
Scientific Reports (2023)
-
Occurrence of antibiotics and bacterial resistance genes in wastewater: resistance mechanisms and antimicrobial resistance control approaches
World Journal of Microbiology and Biotechnology (2022)