Key Points
-
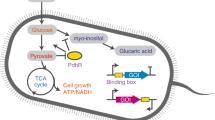
Bacteria adapt readily to many different nutritional environments by altering gene expression in accordance with the availability of particular nutrients. Each metabolic gene or operon is regulated by specific factors that sense the intracellular concentrations of substrates for degradation or the end-products of biosynthesis.
-
Overlying this operon-specific regulation are at least two additional levels of regulation. The first coordinates the expression of groups of operons according to their general function (for example, the use of carbon souces). The second coordinates the expression of large groups of genes according to their impact on the overall balance of central metabolism.
-
In Bacillus subtilis, three global regulatory proteins mediate both function- and metabolism-coordinating gene expression. CcpA and TnrA are the global regulators for carbon metabolism and nitrogen metabolism, respectively, and part of their function is to influence the activity of a third global regulator, CodY.
-
If glucose is available, B. subtilis CodY functions in conjunction with CcpA to repress the use of alternative carbon sources and activate the expression of carbon-overflow pathways.
-
If cells are in a rich medium, CodY also represses the use of alternative nitrogen sources; if the cell has to depend on poor nitrogen sources, repression by CodY is relieved, but at the same time positive regulation of the same genes by TnrA is activated.
-
The activity of CodY as a DNA-binding protein is greatly stimulated by two types of ligands — GTP and the branched-chain amino acids. Both CcpA and TnrA regulate the synthesis of these amino acids, thereby helping to determine the extent to which CodY is active. A fourth global regulatory system, the stringent response to amino-acid deprivation, leads to a drop in the intracellular GTP pool and, therefore, also influences the activity of CodY.
-
In some Gram-positive pathogens, CcpA and CodY proteins control virulence genes as well as metabolism genes, which connects the expression of virulence to the metabolic state of the cell.
Abstract
The remarkable ability of bacteria to adapt efficiently to a wide range of nutritional environments reflects their use of overlapping regulatory systems that link gene expression to intracellular pools of a small number of key metabolites. By integrating the activities of global regulators, such as CcpA, CodY and TnrA, Bacillus subtilis manages traffic through two metabolic intersections that determine the flow of carbon and nitrogen to and from crucial metabolites, such as pyruvate, 2-oxoglutarate and glutamate. Here, the latest knowledge on the control of these key intersections in B. subtilis is reviewed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hammer, B. K. & Swanson, M. S. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33, 721–731 (1999).
Neidhardt, F. C. et al. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology (ASM, Washington DC, 1996).
Deutscher, J., Galinier, A. & Verstraete-Martin, I. in Bacillus subtilis and its Closest Relatives: from Genes to Cells (eds Sonenshein, A. L., Hoch, J. A. & Losick, R. M.) 129–150 (ASM, Washington DC, 2002).
Deutscher, J., Francke, C. & Postma, P. W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70, 939–1031 (2006).
Aymerich, S., Goelzer, A. & Fromion, V. in Global Regulatory Networks in Bacillus subtilis (ed. Fujita, Y.) 39–73 (Transworld Research Network, Trivandum, in the press).
Sonenshein, A. L., Hoch, J. A. & Losick, R. M. (eds) Bacillus subtilis and its Closest Relatives: from Genes to Cells (ASM, Washington DC, 2002).
Presecan-Siedel, E. et al. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J. Bacteriol. 181, 6889–6897 (1999).
Shin, B. S., Choi, S. K. & Park, S. H. Regulation of the Bacillus subtilis phosphotransacetylase gene. J. Biochem. (Tokyo) 126, 333–339 (1999).
Grundy, F. J., Waters, D. A., Allen, S. H. & Henkin, T. M. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J. Bacteriol. 175, 7348–7355 (1993).
Lorca, G. L. et al. Catabolite repression and activation in Bacillus subtilis: dependency on CcpA, HPr, and HprK. J. Bacteriol. 187, 7826–7839 (2005). This paper provides a thorough analysis of the role of CcpA in the control of metabolism.
Moreno, M. S., Schneider, B. L., Maile, R. R., Weyler, W. & Saier, M. H. Jr. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol. Microbiol. 39, 1366–1381 (2001).
Blencke, H. M. et al. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab. Eng. 5, 133–149 (2003).
Grundy, F. J., Turinsky, A. J. & Henkin, T. M. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 176, 4527–4533 (1994).
Shivers, R. P., Dineen, S. S. & Sonenshein, A. L. Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol. Microbiol. 62, 811–822 (2006). This study was the first to demonstrate positive regulation by CodY, and highlighted the interesting and important role of CodY in carbon-overflow pathways.
Ali, N. O., Bignon, J., Rapoport, G. & Debarbouille, M. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J. Bacteriol. 183, 2497–2504 (2001).
Zalieckas, J. M., Wray, L. V. Jr. & Fisher, S. H. Expression of the Bacillus subtilis acsA gene: position and sequence context affect cre-mediated carbon catabolite repression. J. Bacteriol. 180, 6649–6654 (1998).
Warner, J. B. & Lolkema, J. S. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67, 475–490 (2003).
Lulko, A. T., Buist, G., Kok, J. & Kuipers, O. P. Transcriptome analysis of temporal regulation of carbon metabolism by CcpA in Bacillus subtilis reveals additional target genes. J. Mol. Microbiol. Biotechnol. 12, 82–95 (2007).
Ludwig, H., Rebhan, N., Blencke, H. M., Merzbacher, M. & Stulke, J. Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45, 543–553 (2002).
Hanson, K. G., Steinhauer, K., Reizer, J., Hillen, W. & Stulke, J. HPr kinase/phosphatase of Bacillus subtilis: expression of the gene and effects of mutations on enzyme activity, growth and carbon catabolite repression. Microbiology 148, 1805–1811 (2002).
Monedero, V. et al. Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J. 20, 3928–3937 (2001).
Deutscher, J., Kuster, E., Bergstedt, U., Charrier, V. & Hillen, W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 15, 1049–1053 (1995). This paper was the first to establish a connection between CcpA activity, fructose-1,6-bisphosphate and the seryl phosphorylation of HPr.
Galinier, A., Deutscher, J. & Martin-Verstraete, I. Phosphorylation of either Crh or HPr mediates binding of CcpA to the Bacillus subtilis xyn cre and catabolite repression of the xyn operon. J. Mol. Biol. 286, 307–314 (1999).
Seidel, G., Diel, M., Fuchsbauer, N. & Hillen, W. Quantitative interdependence of coeffectors, CcpA and cre in carbon catabolite regulation of Bacillus subtilis. FEBS J. 272, 2566–2577 (2005).
Nicholson, W. L. et al. Catabolite repression-resistant mutations of the Bacillus subtilis alpha-amylase promoter affect transcription levels and are in an operator-like sequence. J. Mol. Biol. 198, 609–618 (1987).
Jault, J. M. et al. The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose-1,6-bisphosphate binding. J. Biol. Chem. 275, 1773–1780 (2000).
Nessler, S. et al. HPr kinase/phosphorylase, the sensor enzyme of catabolite repression in Gram-positive bacteria: structural aspects of the enzyme and the complex with its protein substrate. J. Bacteriol. 185, 4003–4010 (2003).
Schumacher, M. A., Seidel, G., Hillen, W. & Brennan, R. G. Structural mechanism for the fine-tuning of CcpA function by the small molecule effectors glucose 6-phosphate and fructose-1,6-bisphosphate. J. Mol. Biol. 368, 1042–1050 (2007).
Sonenshein, A. L. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8, 203–207 (2005).
Slack, F. J., Serror, P., Joyce, E. & Sonenshein, A. L. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol. Microbiol. 15, 689–702 (1995).
Wray, L. V. Jr., Ferson, A. E. & Fisher, S. H. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J. Bacteriol. 179, 5494–5501 (1997).
Ferson, A. E., Wray, L. V. Jr. & Fisher, S. H. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22, 693–701 (1996).
Fisher, S. H., Rohrer, K. & Ferson, A. E. Role of CodY in regulation of the Bacillus subtilis hut operon. J. Bacteriol. 178, 3779–3784 (1996).
Bergara, F. et al. CodY is a nutritional repressor of flagellar gene expression in Bacillus subtilis. J. Bacteriol. 185, 3118–3126 (2003).
Debarbouille, M., Gardan, R., Arnaud, M. & Rapoport, G. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181, 2059–2066 (1999).
Molle, V. et al. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 185, 1911–1922 (2003).
Serror, P. & Sonenshein, A. L. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178, 5910–5915 (1996).
Inaoka, T. & Ochi, K. RelA protein is involved in induction of genetic competence in certain Bacillus subtilis strains by moderating the level of intracellular GTP. J. Bacteriol. 184, 3923–3930 (2002). The first direct evidence that the activation of RelA changes the activity of CodY, which brought two major regulons together.
Inaoka, T., Takahashi, K., Ohnishi-Kameyama, M., Yoshida, M. & Ochi, K. Guanine nucleotides guanosine 5′-diphosphate 3′-diphosphate and GTP co-operatively regulate the production of an antibiotic bacilysin in Bacillus subtilis. J. Biol. Chem. 278, 2169–2176 (2003).
Ratnayake-Lecamwasam, M., Serror, P., Wong, K. W. & Sonenshein, A. L. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15, 1093–1103 (2001). The first demonstration that GTP binds to CodY and increases its activity as a transcription factor.
Shivers, R. P. & Sonenshein, A. L. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol. Microbiol. 53, 599–611 (2004). The first evidence that BCAAs interact directly with CodY and increase its affinity for DNA.
Guedon, E., Sperandio, B., Pons, N., Ehrlich, S. D. & Renault, P. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology 151, 3895–3909 (2005).
den Hengst, C. D. et al. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 280, 34332–34342 (2005).
Jin, S. & Sonenshein, A. L. Identification of two distinct Bacillus subtilis citrate synthase genes. J. Bacteriol. 176, 4669–4679 (1994).
Jin, S., De Jesus-Berrios, M. & Sonenshein, A. L. A Bacillus subtilis malate dehydrogenase gene. J. Bacteriol. 178, 560–563 (1996).
Jin, S. & Sonenshein, A. L. Transcriptional regulation of Bacillus subtilis citrate synthase genes. J. Bacteriol. 176, 4680–4690 (1994).
Dingman, D. W. & Sonenshein, A. L. Purification of aconitase from Bacillus subtilis and correlation of its N-terminal amino acid sequence with the sequence of the citB gene. J. Bacteriol. 169, 3062–3067 (1987).
Jourlin-Castelli, C., Mani, N., Nakano, M. M. & Sonenshein, A. L. CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J. Mol. Biol. 295, 865–878 (2000).
Kim, H. J., Roux, A. & Sonenshein, A. L. Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol. Microbiol. 45, 179–190 (2002).
Kim, H. J., Jourlin-Castelli, C., Kim, S. I. & Sonenshein, A. L. Regulation of the Bacillus subtilis ccpC gene by ccpA and ccpC. Mol. Microbiol. 43, 399–410 (2002).
Kim, H. J., Mittal, M. & Sonenshein, A. L. CcpC-dependent regulation of citB and lmo0847 in Listeria monocytogenes. J. Bacteriol. 188, 179–190 (2006).
Blencke, H. M. et al. Regulation of citB expression in Bacillus subtilis: integration of multiple metabolic signals in the citrate pool and by the general nitrogen regulatory system. Arch. Microbiol. 185, 136–146 (2006).
Jobe, A. & Bourgeois, S. lac repressor-operator interaction. VI. The natural inducer of the lac operon. J. Mol. Biol. 69, 397–408 (1972).
Schlesinger, S., Scotto, P. & Magasanik, B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J. Biol. Chem. 240, 4331–4337 (1965).
Kim, H. J. et al. Complex regulation of the Bacillus subtilis aconitase gene. J. Bacteriol. 185, 1672–1680 (2003).
Soga, T., Ohashi, Y., Ueno, Y., Naraoka, H., Tomita, M. & Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2, 488–494 (2003).
Fisher, S. H. & Magasanik, B. 2-Ketoglutarate and the regulation of aconitase and histidase formation in Bacillus subtilis. J. Bacteriol. 158, 379–382 (1984).
Bohannon, D. E. & Sonenshein, A. L. Positive regulation of glutamate biosynthesis in Bacillus subtilis. J. Bacteriol. 171, 4718–4727 (1989).
Belitsky, B. R., Janssen, P. J. & Sonenshein, A. L. Sites required for GltC-dependent regulation of Bacillus subtilis glutamate synthase expression. J. Bacteriol. 177, 5686–5695 (1995).
Picossi, S., Belitsky, B. R. & Sonenshein, A. L. Molecular mechanism of the regulation of Bacillus subtilis gltAB expression by GltC. J. Mol. Biol. 365, 1298–1313 (2007).
Commichau, F. M., Herzberg, C., Tripal, P., Valerius, O. & Stulke, J. A regulatory protein–protein interaction governs glutamate biosynthesis in Bacillus subtilis: the glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol. Microbiol. 65, 642–654 (2007). This recent paper shows that glutamate dehydrogenase interacts with GltC, thereby reducing glutamate synthesis.
Wray, L. V. Jr., Ferson, A. E., Rohrer, K. & Fisher, S. H. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc. Natl Acad. Sci. USA 93, 8841–8845 (1996).
Zalieckas, J. M., Wray, L. V. Jr. & Fisher, S. H. trans-acting factors affecting carbon catabolite repression of the hut operon in Bacillus subtilis. J. Bacteriol. 181, 2883–2888 (1999).
Yoshida, K. et al. Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol. Microbiol. 49, 157–165 (2003).
Wray, L. V. Jr., Zalieckas, J. M. & Fisher, S. H. Bacillus subtilis glutamine synthetase controls gene expression through a protein–protein interaction with transcription factor TnrA. Cell 107, 427–435 (2001). The authors reveal that TnrA, the global regulator of nitrogen metabolism in B. subtilis , is inactivated by direct interaction with a complex of glutamine synthetase and glutamine.
Belitsky, B. R. & Sonenshein, A. L. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180, 6298–6305 (1998).
Gardan, R., Rapoport, G. & Debarbouille, M. Role of the transcriptional activator RocR in the arginine-degradation pathway of Bacillus subtilis. Mol. Microbiol. 24, 825–837 (1997).
Belitsky, B. R. & Sonenshein, A. L. An enhancer element located downstream of the major glutamate dehydrogenase gene of Bacillus subtilis. Proc. Natl Acad. Sci. USA 96, 10290–10295 (1999).
Ali, N. O. et al. Specificity of the interaction of RocR with the rocG–rocA intergenic region in Bacillus subtilis. Microbiology 149, 739–750 (2003).
Belitsky, B. R., Kim, H. J. & Sonenshein, A. L. CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression. J. Bacteriol. 186, 3392–3398 (2004).
Faires, N. et al. The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1, 141–148 (1999).
Wacker, I. et al. The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149, 3001–3009 (2003).
Belitsky, B. R. & Sonenshein, A. L. Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase. J. Bacteriol. 186, 3399–3407 (2004).
Wray, L. V. Jr., Pettengill, F. K. & Fisher, S. H. Catabolite repression of the Bacillus subtilis hut operon requires a cis-acting site located downstream of the transcription initiation site. J. Bacteriol. 176, 1894–1902 (1994).
Strauch, M. A., Aronson, A. I., Brown, S. W., Schreier, H. J. & Sonenshein, A. L. Sequence of the Bacillus subtilis glutamine synthetase gene region. Gene 71, 257–265 (1988).
Brown, S. W. & Sonenshein, A. L. Autogenous regulation of the Bacillus subtilis glnRA operon. J. Bacteriol. 178, 2450–2454 (1996).
Nakano, Y. & Kimura, K. Purification and characterization of a repressor for the Bacillus cereus glnRA operon. J. Biochem. (Tokyo) 109, 223–228 (1991).
Brandenburg, J. L. et al. Roles of PucR, GlnR, and TnrA in regulating expression of the Bacillus subtilis ure P3 promoter. J. Bacteriol. 184, 6060–6064 (2002).
Zalieckas, J. M., Wray, L. V. Jr. & Fisher, S. H. Cross-regulation of the Bacillus subtilis glnRA and tnrA genes provides evidence for DNA binding site discrimination by GlnR and TnrA. J. Bacteriol. 188, 2578–2585 (2006).
Schreier, H. J., Brown, S. W., Hirschi, K. D., Nomellini, J. F. & Sonenshein, A. L. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J. Mol. Biol. 210, 51–63 (1989).
Fisher, S. H. Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference! Mol. Microbiol. 32, 223–232 (1999).
Fisher, S. H. & Wray, L. V. Jr. Feedback-resistant mutations in Bacillus subtilis glutamine synthetase are clustered in the active site. J. Bacteriol. 188, 5966–5974 (2006).
Kloosterman, T. G. et al. Regulation of glutamine and glutamate metabolism by GlnR and GlnA in Streptococcus pneumoniae. J. Biol. Chem. 281, 25097–25109 (2006).
Sauer, U. & Eikmanns, B. J. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 29, 765–794 (2005). A landmark paper that analyses the physiological consequences of metabolite flux.
Fink, P. S. in Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology and Molecular Genetics (eds Sonenshein, A. L., Hoch, J. A. & Losick, R.) 307–317 (ASM, Washington DC, 1993).
Ludwig, H., Meinken, C., Matin, A. & Stulke, J. Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant. J. Bacteriol. 184, 5174–5178 (2002).
Tojo, S. et al. Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY and TnrA. Mol. Microbiol. 56, 1560–1573 (2005). This paper reported simultaneously with reference 88 that CodY and CcpA have antagonistic effects on ilvB transcription, so establishing a crucial interaction between the two regulons.
Shivers, R. P. & Sonenshein, A. L. Bacillus subtilis ilvB operon: an intersection of global regulons. Mol. Microbiol. 56, 1549–1559 (2005).
Tojo, S. et al. Negative transcriptional regulation of the ilv-leu operon for biosynthesis of branched-chain amino acids through the Bacillus subtilis global regulator TnrA. J. Bacteriol. 186, 7971–7979 (2004).
Calvo, J. M. & Matthews, R. G. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol. Rev. 58, 466–490 (1994).
Paul, L., Mishra, P. K., Blumenthal, R. M. & Matthews, R. G. Integration of regulatory signals through involvement of multiple global regulators: control of the Escherichia coli gltBDF operon by Lrp, IHF, Crp, and ArgR. BMC Microbiol. 7, 2 (2007).
Tani, T. H., Khodursky, A., Blumenthal, R. M., Brown, P. O. & Matthews, R. G. Adaptation to famine: a family of stationary-phase genes revealed by microarray analysis. Proc. Natl Acad. Sci. USA 99, 13471–13476 (2002).
de Mendoza, D. in Bacillus subtilis and its Closest Relatives: From Genes to Cells (eds Sonenshein, A. L., Hoch, J. A. & Losick, R.) 43–55 (ASM, Washington DC, 2002).
Cashel, M., Gentry, D. R., Hernandez, V. J. & Vinella, D. in Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology (eds Neidhardt, F. C. et al.) 1458–1496 (ASM, Washington DC, 1996).
Wendrich, T. M. & Marahiel, M. A. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26, 65–79 (1997).
Eymann, C., Homuth, G., Scharf, C. & Hecker, M. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 184, 2500–2520 (2002).
Ochi, K., Kandala, J. C. & Freese, E. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J. Biol. Chem. 256, 6866–6875 (1981).
Lopez, J. M., Dromerick, A. & Freese, E. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J. Bacteriol. 146, 605–613 (1981).
Krasny, L. & Gourse, R. L. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 23, 4473–4483 (2004).
Giammarinaro, P. & Paton, J. C. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70, 5454–5461 (2002). One of the earliest demonstrations of a role for CcpA in pathogenesis.
Iyer, R., Baliga, N. S. & Camilli, A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187, 8340–8349 (2005).
Asanuma, N., Yoshii, T. & Hino, T. Molecular characterization of CcpA and involvement of this protein in transcriptional regulation of lactate dehydrogenase and pyruvate formate-lyase in the ruminal bacterium Streptococcus bovis. Appl. Environ. Microbiol. 70, 5244–5251 (2004).
Luesink, E. J., van Herpen, R. E., Grossiord, B. P., Kuipers, O. P. & de Vos, W. M. Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol. Microbiol. 30, 789–798 (1998).
Behari, J. & Youngman, P. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J. Bacteriol. 180, 6316–6324 (1998).
Mertins, S. et al. Interference of components of the phosphoenolpyruvate phosphotransferase system with the central virulence gene regulator PrfA of Listeria monocytogenes. J. Bacteriol. 189, 473–490 (2007).
Seidl, K. et al. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50, 1183–1194 (2006).
Kreft, J. & Vazquez-Boland, J. A. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 291, 145–157 (2001).
Herro, R. et al. How seryl-phosphorylated HPr inhibits PrfA, a transcription activator of Listeria monocytogenes virulence genes. J. Mol. Microbiol. Biotechnol. 9, 224–234 (2005).
Jankovic, I. & Bruckner, R. Carbon catabolite repression by the catabolite control protein CcpA in Staphylococcus xylosus. J. Mol. Microbiol. Biotechnol. 4, 309–314 (2002).
Varga, J., Stirewalt, V. L. & Melville, S. B. The CcpA protein is necessary for efficient sporulation and enterotoxin (cpe) regulation in Clostridium perfringens. J. Bacteriol. 186, 5221–5229 (2004).
Bennett, H. J. et al. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63, 1453–1467 (2007).
Malke, H., Steiner, K., McShan, W. M. & Ferretti, J. J. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int. J. Med. Microbiol. 296, 259–275 (2006). The first published evidence that a CodY protein in a pathogenic bacterium is involved in the regulation of virulence genes.
Petranovic, D. et al. Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol. Microbiol. 53, 613–621 (2004).
den Hengst, C. D., Groeneveld, M., Kuipers, O. P. & Kok, J. Identification and functional characterization of the Lactococcus lactis CodY-regulated branched-chain amino acid permease BcaP (CtrA). J. Bacteriol. 188, 3280–3289 (2006).
Chambellon, E. & Yvon, M. CodY-regulated aminotransferases AraT and BcaT play a major role in the growth of Lactococcus lactis in milk by regulating the intracellular pool of amino acids. Appl. Environ. Microbiol. 69, 3061–3068 (2003).
Guedon, E., Serror, P., Ehrlich, S. D., Renault, P. & Delorme, C. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40, 1227–1239 (2001). The first evidence that CodY responds in vivo to BCAAs.
den Hengst, C. D. et al. Probing direct interactions between CodY and the oppD promoter of Lactococcus lactis. J. Bacteriol. 187, 512–521 (2005).
Malke, H. & Ferretti, J. J. CodY-affected transcriptional gene expression of Streptococcus pyogenes during growth in human blood. J. Med. Microbiol. 56, 707–714 (2007).
Mani, N. & Dupuy, B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl Acad. Sci. USA 98, 5844–5849 (2001).
Mani, N. et al. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 184, 5971–5978 (2002).
Hundsberger, T. et al. Transcription analysis of the genes tcdA–E of the pathogenicity locus of Clostridium difficile. Eur. J. Biochem. 244, 735–742 (1997).
Dupuy, B. & Sonenshein, A. L. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27, 107–120 (1998).
Dineen, S. S., Villapakkam, A., Nordman, J. & Sonenshein, A. L. Repression of Clostridium difficile toxin genes by CodY. Mol. Microbiol. 66, 206–219 (2007). This paper presents the first example of direct interaction between CodY and a virulence-gene locus.
Tu Quoc, P. H. et al. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect. Immun. 75, 1079–1088 (2007).
Taylor, C. M. et al. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol. 184, 621–628 (2002).
Acknowledgements
The author thanks the anonymous reviewers for many helpful suggestions. The unpublished work cited in this Review was supported by research grants from the US Public Health Service (GM042219, GM036718 and AI057637).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez Genome Project
Entrez Protein
FURTHER INFORMATION
Glossary
- Glycolysis
-
The metabolic pathway that converts glucose into pyruvate, with the concomitant production of ATP and NADH.
- Pentose-phosphate pathway
-
The metabolic pathway by which glucose-6-phosphate is oxidized to ribose-5-phosphate, with the concomitant production of NADPH.
- Citric acid cycle
-
The metabolic pathway that oxidizes acetyl CoA to carbon dioxide.
- 2-oxoglutarate–glutamate–glutamine cycle
-
The metabolic pathway that connects carbon and nitrogen metabolism and permits ammonium ions to be incorporated into organic molecules.
- Global regulator
-
A protein that controls many genes and operons in response to a specific signal.
- Sporulation
-
A developmental programme in some microorganisms in response to unfavourable environmental conditions that results in spores that are highly resistant to environmental stresses.
- LacI protein family
-
The proteins that are related in sequence and function to the classical repressor of the E. coli lac operon.
- Phosphoenolpyruvate-dependent phosphotransferase transport system
-
A multi-protein phosphorelay system that couples the phosphorylation of sugars to their transport across the cytoplasmic membrane.
- Competence
-
The ability of bacteria to take up extracellular DNA.
- Homolactic fermentation
-
The production of lactic acid (lactate) as the sole pyruvate-derived product during growth on sugars, such as glucose.
- Two-component regulatory system
-
A system that responds to an environmental stimulus and regulates gene expression accordingly. Composed of a histidine-kinase sensor, usually situated in the outer membrane, that phosphorylates a response regulator in the cytoplasm, which, in turn, activates transcription from selected promoters.
Rights and permissions
About this article
Cite this article
Sonenshein, A. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol 5, 917–927 (2007). https://doi.org/10.1038/nrmicro1772
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1772
This article is cited by
-
Transcriptional modulation of the global regulator CodY using a conditional CRISPRi system in Bacillus licheniformis
Systems Microbiology and Biomanufacturing (2024)
-
Genome-wide mapping of GlnR-binding sites reveals the global regulatory role of GlnR in controlling the metabolism of nitrogen and carbon in Paenibacillus polymyxa WLY78
BMC Genomics (2023)
-
Mechanisms of probiotic Bacillus against enteric bacterial infections
One Health Advances (2023)
-
Functional characterization of the phosphotransferase system in Parageobacillus thermoglucosidasius
Scientific Reports (2023)
-
Engineering of global transcription factors in Bacillus, a genetic tool for increasing product yields: a bioprocess overview
World Journal of Microbiology and Biotechnology (2023)