Key Points
-
The emergence of drug resistance in pathogenic microorganisms provides an excellent example of evolution that has had profound consequences for human health. As the evolution of drug resistance is outpacing the development of new antimicrobial agents, it is now crucial to understand the evolutionary mechanisms that are involved in order to maintain effective therapeutic strategies.
-
Fungal pathogens pose a particularly acute challenge, owing to the limited number of clinically useful antifungal drugs that are available and the rising incidence and mortality of infections with Candida albicans, Aspergillus fumigatus and Cryptococcus neoformans. As tractable model eukaryotes, fungi also provide powerful model systems for the study of evolution, cellular signalling and the genetic architecture of complex traits.
-
This article focuses on the mechanisms that enable the evolution of fungal drug resistance by modulating the trajectory from genotype to phenotype, with an emphasis on the central role of the molecular chaperone heat shock protein 90 (Hsp90). Hsp90 regulates the form and function of diverse signal transducers and can function as a capacitor for the storage of genetic variation in a silent state that can be released in response to environmental stress.
-
Hsp90 enables the rapid evolution of resistance to the widely used azole antifungal drugs in Saccharomyces cerevisiae and C. albicans, and is also required for the phenotypic consequences of resistance that is acquired owing to diverse mutations in the genome. The role of Hsp90 in azole resistance is to enable crucial cellular stress responses to the membrane stress that is exerted by the azoles.
-
In Aspergillus species, Hsp90 potentiates basal resistance to the only new class of antifungal drugs to reach the clinic in decades, the echinocandins. The role of Hsp90 in echinocandin resistance is to enable specific cellular stress responses to the cell-wall stress that is exerted by the echinocandins.
-
The central mediator of Hsp90-dependent drug resistance is calcineurin, a key regulator of cellular signalling that requires Hsp90 to maintain its stable form and function. Inhibition of calcineurin with FK506 or cyclosporin A phenocopies the inhibition of Hsp90 with geldanamycin or radicicol, thereby reducing the drug resistance of fungi that are separated by ∼1 billion years of evolution. Drug resistance can evolve from Hsp90-dependence to Hsp90-independence by the accumulation of additional mutations that allow the cell to bypass the stress that is exerted by the antifungal drug.
-
Although Hsp90 provides one of the best examples of an explicit mechanism that can alter the relationship between genotype and phenotype and potentiate the evolution of drug resistance, there are other ways in which alterations in the cellular state can affect resistance phenotypes. Fungal prions — proteins that can adopt an altered conformation that is self-perpetuating and are transmitted as a protein-based element of inheritance — can have a profound impact on resistance phenotypes, as can elaboration of the complex architecture of fungal biofilms.
-
The role of Hsp90 in the emergence and maintenance of fungal drug resistance suggests a promising new combination strategy for treating fungal infections. Pharmacological inhibitors of Hsp90 that are well tolerated in humans can block the evolution of drug resistance and abrogate drug resistance in diverse fungal pathogens, and thus may render resistant pathogens responsive to treatment.
Abstract
The emergence of drug resistance in pathogenic microorganisms provides an excellent example of microbial evolution that has had profound consequences for human health. The widespread use of antimicrobial agents in medicine and agriculture exerts strong selection for the evolution of drug resistance. Selection acts on the phenotypic consequences of resistance mutations, which are influenced by the genetic variation in particular genomes. Recent studies have revealed a mechanism by which the molecular chaperone heat shock protein 90 (Hsp90) can alter the relationship between genotype and phenotype in an environmentally contingent manner, thereby 'sculpting' the course of evolution. Harnessing Hsp90 holds great promise for treating life-threatening infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anderson, J. B. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nature Rev. Microbiol. 3, 547–556 (2005). An excellent introduction to fungal drug resistance from an evolutionary perspective.
Antonovics, J. et al. Evolution by any other name: antibiotic resistance and avoidance of the e-word. PLoS Biol. 5, e30 (2007).
Cowen, L. E. Predicting the emergence of resistance to antifungal drugs. FEMS Microbiol. Lett. 204, 1–7 (2001).
Cowen, L. E., Anderson, J. B. & Kohn, L. M. Evolution of drug resistance in Candida albicans. Annu. Rev. Microbiol. 56, 139–165 (2002).
Levin, B. R., Lipsitch, M. & Bonhoeffer, S. Population biology, evolution, and infectious disease: convergence and synthesis. Science 283, 806–809 (1999).
Riley, M. A. & Wertz, J. E. Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56, 117–137 (2002).
Wright, G. D. The antibiotic resistome: the nexus of chemical and genetic diversity. Nature Rev. Microbiol. 5, 175–186 (2007).
Yim, G., Wang, H. H. & Davies, J. Antibiotics as signalling molecules. Phil. Trans. R. Soc. Lond. B 362, 1195–1200 (2007).
Palumbi, S. R. Humans as the world's greatest evolutionary force. Science 293, 1786–1790 (2001).
Pfaller, M. A. & Diekema, D. J. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 42, 4419–4431 (2004).
White, T. C., Marr, K. A. & Bowden, R. A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11, 382–402 (1998).
Alekshun, M. N. & Levy, S. B. Molecular mechanisms of antibacterial multidrug resistance. Cell 128, 1037–1050 (2007).
Hastings, P. J., Rosenberg, S. M. & Slack, A. Antibiotic-induced lateral transfer of antibiotic resistance. Trends Microbiol. 12, 401–404 (2004).
Rambaut, A., Posada, D., Crandall, K. A. & Holmes, E. C. The causes and consequences of HIV evolution. Nature Rev. Genet. 5, 52–61 (2004).
Woodford, N. & Ellington, M. J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 13, 5–18 (2007).
Maisnier-Patin, S. & Andersson, D. I. Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res. Microbiol. 155, 360–369 (2004).
Perlstein, E. O. et al. Revealing complex traits with small molecules and naturally recombinant yeast strains. Chem. Biol. 13, 319–327 (2006).
Perlstein, E. O., Ruderfer, D. M., Roberts, D. C., Schreiber, S. L. & Kruglyak, L. Genetic basis of individual differences in the response to small-molecule drugs in yeast. Nature Genet. 39, 496–502 (2007).
Baldauf, S. L., Roger, A. J., Wenk-Siefert, I. & Doolittle, W. F. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290, 972–977 (2000).
Odds, F. C., Brown, A. J. & Gow, N. A. Antifungal agents: mechanisms of action. Trends Microbiol. 11, 272–279 (2003).
Fraser, J. A. et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437, 1360–1364 (2005).
Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Pfaller, M. A. & Diekema, D. J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163 (2007).
McNeil, M. M. et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin. Infect. Dis. 33, 641–647 (2001).
Wilson, L. S. et al. The direct cost and incidence of systemic fungal infections. Value Health 5, 26–34 (2002).
Elena, S. F. & Lenski, R. E. Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nature Rev. Genet. 4, 457–469 (2003).
Zeyl, C. Experimental evolution with yeast. FEMS Yeast Res. 6, 685–691 (2006).
Bahn, Y. S. et al. Sensing the environment: lessons from fungi. Nature Rev. Microbiol. 5, 57–69 (2007).
Boone, C., Bussey, H. & Andrews, B. J. Exploring genetic interactions and networks with yeast. Nature Rev. Genet. 8, 437–449 (2007).
Hartman, J. L., Garvik, B. & Hartwell, L. Principles for the buffering of genetic variation. Science 291, 1001–1004 (2001). Provides an important perspective on the complex relationship between genotype and phenotype and mechanisms of buffering variation in eukaryotic genomes.
Rockman, M. V. & Kruglyak, L. Genetics of global gene expression. Nature Rev. Genet. 7, 862–872 (2006).
Steinmetz, L. M. & Davis, R. W. Maximizing the potential of functional genomics. Nature Rev. Genet. 5, 190–201 (2004).
Lupetti, A., Danesi, R., Campa, M., Del Tacca, M. & Kelly, S. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8, 76–81 (2002).
Turner, M. S., Drew, R. H. & Perfect, J. R. Emerging echinocandins for treatment of invasive fungal infections. Expert Opin. Emerg. Drugs 11, 231–250 (2006).
Rex, J. H. & Pfaller, M. A. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35, 982–989 (2002).
Sanglard, D. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 5, 379–385 (2002).
Sanglard, D. & Odds, F. C. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2, 73–85 (2002).
Coste, A. et al. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 6, 1889–1904 (2007).
da Silva Ferreira, M. E. et al. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob. Agents Chemother. 48, 4405–4413 (2004).
Nascimento, A. M. et al. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 47, 1719–1726 (2003).
Pasrija, R., Banerjee, D. & Prasad, R. Structure and function analysis of CaMdr1p, a major facilitator superfamily antifungal efflux transporter protein of Candida albicans: identification of amino acid residues critical for drug/H+ transport. Eukaryot. Cell 6, 443–453 (2007).
Perea, S. et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45, 2676–2684 (2001).
Chamilos, G. & Kontoyiannis, D. P. Update on antifungal drug resistance mechanisms of Aspergillus fumigatus. Drug Resist. Updat. 8, 344–358 (2005).
Coste, A. et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172, 2139–2156 (2006).
Coste, A. T., Karababa, M., Ischer, F., Bille, J. & Sanglard, D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3, 1639–1652 (2004).
Hiller, D., Stahl, S. & Morschhauser, J. Multiple cis-acting sequences mediate upregulation of the MDR1 efflux pump in a fluconazole-resistant clinical Candida albicans isolate. Antimicrob. Agents Chemother. 50, 2300–2308 (2006).
Liu, T. T. et al. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot. Cell 6, 2122–2138 (2007).
Morschhauser, J. et al. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3, e164 (2007).
Riggle, P. J. & Kumamoto, C. A. Transcriptional regulation of MDR1, encoding a drug efflux determinant, in fluconazole-resistant Candida albicans strains through an Mcm1p binding site. Eukaryot. Cell 5, 1957–1968 (2006).
Rognon, B., Kozovska, Z., Coste, A. T., Pardini, G. & Sanglard, D. Identification of promoter elements responsible for the regulation of MDR1 from Candida albicans, a major facilitator transporter involved in azole resistance. Microbiology 152, 3701–3722 (2006).
Selmecki, A., Forche, A. & Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313, 367–370 (2006). Established a novel mechanism of fungal drug resistance that involves aneuploidy and isochromosome formation; also showcased the genomic plasticity that underpins phenotypic variability.
Niimi, K. et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob. Agents Chemother. 50, 1148–1155 (2006).
Perlin, D. S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10, 121–130 (2007).
MacPherson, S. et al. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 49, 1745–1752 (2005).
Silver, P. M., Oliver, B. G. & White, T. C. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 3, 1391–1397 (2004).
Giaever, G. et al. Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc. Natl Acad. Sci. USA 101, 793–798 (2004).
Hu, W. et al. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3, e24 (2007).
Xu, D. et al. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 3, e92 (2007).
White, T. C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41, 1482–1487 (1997).
Anderson, J. B., Ricker, N. & Sirjusingh, C. Antagonism between two mechanisms of antifungal drug resistance. Eukaryot. Cell 5, 1243–1251 (2006).
Cowen, L. E., Kohn, L. M. & Anderson, J. B. Divergence in fitness and evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 183, 2971–2978 (2001).
Cowen, L. E. et al. Evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 182, 1515–1522 (2000).
Cowen, L. E. et al. Population genomics of drug resistance in Candida albicans. Proc. Natl Acad. Sci. USA 99, 9284–9289 (2002).
Cowen, L. E., Carpenter, A. E., Matangkasombut, O., Fink, G. R. & Lindquist, S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 5, 2184–2188 (2006).
Cowen, L. E. & Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309, 2185–2189 (2005). Describes a new mechanism by which Hsp90 can enable the evolution of fungal drug resistance and modulate the relationship between genotype and phenotype.
Pearl, L. H. & Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75, 271–294 (2006).
Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. 59, 1640–1648 (2002).
Pratt, W. B. & Toft, D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 228, 111–133 (2003).
Young, J. C., Moarefi, I. & Hartl, F. U. Hsp90: a specialized but essential protein-folding tool. J. Cell. Biol. 154, 267–273 (2001).
Chang, H. C. & Lindquist, S. Conservation of Hsp90 macromolecular complexes in Saccharomyces cerevisiae. J. Biol. Chem. 269, 24983–24988 (1994).
Nathan, D. F., Vos, M. H. & Lindquist, S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc. Natl Acad. Sci. USA 94, 12949–12956 (1997).
Roe, S. M. et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266 (1999).
Whitesell, L., Mimnaugh, E. G., De Costa, B., Myers, C. E. & Neckers, L. M. Inhibition of heat shock protein HSP90–pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl Acad. Sci. USA 91, 8324–8328 (1994).
Sangster, T. A., Lindquist, S. & Queitsch, C. Under cover: causes, effects and implications of Hsp90-mediated genetic capacitance. Bioessays 26, 348–362 (2004).
Queitsch, C., Sangster, T. A. & Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 417, 618–624 (2002). Demonstrated that the molecular chaperone Hsp90 buffers development from the destabilizing effects of stochastic processes and also buffers the expression of genetic variation in A. thaliana.
Rutherford, S. L. & Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 396, 336–342 (1998). This groundbreaking study established that Hsp90 could buffer the expression of genetic variation in Drosophila melanogaster , and therefore allow cryptic variation to accumulate until revealed by environmental stress.
Sollars, V. et al. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nature Genet. 33, 70–74 (2003).
Zhao, R. et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell 120, 715–727 (2005). Provides a high-resolution map of the physical, genetic and chemical–genetic interactions of Hsp90 in S. cerevisiae.
Ruden, D. M., Garfinkel, M. D., Sollars, V. E. & Lu, X. Waddington's widget: Hsp90 and the inheritance of acquired characters. Semin. Cell Dev. Biol. 14, 301–310 (2003).
Rutherford, S. L. Between genotype and phenotype: protein chaperones and evolvability. Nature Rev. Genet. 4, 263–274 (2003).
Bergman, A. & Siegal, M. L. Evolutionary capacitance as a general feature of complex gene networks. Nature 424, 549–552 (2003).
Hughes, T. R. et al. Functional discovery via a compendium of expression profiles. Cell 102, 109–126 (2000).
Kirschner, M. & Gerhart, J. Evolvability. Proc. Natl Acad. Sci. USA 95, 8420–8427 (1998). An insightful perspective on evolvability 2014 the capacity to generate heritable phenotypic variation.
Fox, D. S. & Heitman, J. Good fungi gone bad: the corruption of calcineurin. Bioessays 24, 894–903 (2002).
Heitman, J. Cell biology. A fungal Achilles' heel. Science 309, 2175–2176 (2005).
Steinbach, W. J., Reedy, J. L., Cramer, R. A. Jr, Perfect, J. R. & Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nature Rev. Microbiol. 5, 418–430 (2007). An excellent review of calcineurin biology in fungal pathogens and our potential to harness this cellular regulator in the treatment of fungal infections.
Cruz, M. C. et al. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21, 546–559 (2002).
Sanglard, D., Ischer, F., Marchetti, O., Entenza, J. & Bille, J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48, 959–976 (2003).
Steinbach, W. J. et al. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48, 1664–1669 (2004).
Imai, J. & Yahara, I. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol. Cell. Biol. 20, 9262–9270 (2000).
Kumar, R., Musiyenko, A. & Barik, S. Plasmodium falciparum calcineurin and its association with heat shock protein 90: mechanisms for the antimalarial activity of cyclosporin A and synergism with geldanamycin. Mol. Biochem. Parasitol. 141, 29–37 (2005).
Someren, J. S., Faber, L. E., Klein, J. D. & Tumlin, J. A. Heat shock proteins 70 and 90 increase calcineurin activity in vitro through calmodulin-dependent and independent mechanisms. Biochem. Biophys. Res. Commun. 260, 619–625 (1999).
Karababa, M. et al. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59, 1429–1451 (2006).
Santos, M. & de Larrinoa, I. F. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 48, 88–100 (2005).
Yoshimoto, H. et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277, 31079–31088 (2002).
Onyewu, C., Wormley, F. L. Jr, Perfect, J. R. & Heitman, J. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 72, 7330–7333 (2004).
Heath, V. L., Shaw, S. L., Roy, S. & Cyert, M. S. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3, 695–704 (2004).
McClellan, A. J. et al. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 131, 121–135 (2007).
Siegal, M. L., Promislow, D. E. & Bergman, A. Functional and evolutionary inference in gene networks: does topology matter? Genetica 129, 83–103 (2007).
Waddington, C. H. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (1942).
Waddington, C. H. Genetic assimilation of an acquired character. Evolution 7, 118–126 (1953).
Hasday, J. D., Fairchild, K. D. & Shanholtz, C. The role of fever in the infected host. Microbes Infect. 2, 1891–1904 (2000).
True, H. L., Berlin, I. & Lindquist, S. L. Epigenetic regulation of translation reveals hidden genetic variation to produce complex traits. Nature 431, 184–187 (2004).
True, H. L. & Lindquist, S. L. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407, 477–483 (2000). Describes how the yeast prion [PSI+] can uncover cryptic genetic variation and generate new heritable phenotypes.
Blankenship, J. R. & Mitchell, A. P. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9, 588–594 (2006).
Nett, J. & Andes, D. Candida albicans biofilm development, modeling a host–pathogen interaction. Curr. Opin. Microbiol. 9, 340–345 (2006).
Nobile, C. J. & Mitchell, A. P. Genetics and genomics of Candida albicans biofilm formation. Cell. Microbiol. 8, 1382–1391 (2006).
Wargo, M. J. & Hogan, D. A. Fungal–bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 9, 359–364 (2006).
d'Enfert, C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr. Drug Targets 7, 465–470 (2006).
Nett, J. et al. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51, 510–520 (2007).
Andes, D. et al. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72, 6023–6031 (2004).
Baillie, G. S. & Douglas, L. J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42, 1900–1905 (1998).
Halme, A., Bumgarner, S., Styles, C. & Fink, G. R. Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell 116, 405–415 (2004).
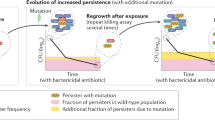
LaFleur, M. D., Kumamoto, C. A. & Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50, 3839–3846 (2006).
Levin, B. R. & Rozen, D. E. Non-inherited antibiotic resistance. Nature Rev. Microbiol. 4, 556–562 (2006).
Lewis, K. Persister cells, dormancy and infectious disease. Nature Rev. Microbiol. 5, 48–56 (2007).
Avery, S. V. Microbial cell individuality and the underlying sources of heterogeneity. Nature Rev. Microbiol. 4, 577–587 (2006).
Keith, C. T., Borisy, A. A. & Stockwell, B. R. Multicomponent therapeutics for networked systems. Nature Rev. Drug Discov. 4, 71–78 (2005).
McLellan, C. A. et al. A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol. 145, 174–182 (2007).
Lehar, J. et al. Chemical combination effects predict connectivity in biological systems. Mol. Syst. Biol. 3, 80 (2007).
Yeh, P., Tschumi, A. I. & Kishony, R. Functional classification of drugs by properties of their pairwise interactions. Nature Genet. 38, 489–494 (2006).
Blankson, J. N., Persaud, D. & Siliciano, R. F. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53, 557–593 (2002).
Griffith, K. S., Lewis, L. S., Mali, S. & Parise, M. E. Treatment of malaria in the United States: a systematic review. JAMA 297, 2264–2277 (2007).
Mukherjee, P. K., Sheehan, D. J., Hitchcock, C. A. & Ghannoum, M. A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18, 163–194 (2005).
Wright, G. D. & Sutherland, A. D. New strategies for combating multidrug-resistant bacteria. Trends Mol. Med. 13, 260–267 (2007).
Kwak, E. L., Clark, J. W. & Chabner, B. Targeted agents: the rules of combination. Clin. Cancer Res. 13, 5232–5237 (2007).
Smith, P. A. & Romesberg, F. E. Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nature Chem. Biol. 3, 549–556 (2007).
Chait, R., Craney, A. & Kishony, R. Antibiotic interactions that select against resistance. Nature 446, 668–671 (2007). Demonstrates an intriguing feature of the fitness landscape 2014 specific drug interactions can select against resistant populations.
Johnson, M. D. & Perfect, J. R. Combination antifungal therapy: what can and should we expect? Bone Marrow Transplant. 40, 297–306 (2007).
Blankenship, J. R., Steinbach, W. J., Perfect, J. R. & Heitman, J. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4, 192–199 (2003).
Pachl, J. et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42, 1404–1413 (2006).
Chiosis, G. Targeting chaperones in transformed systems — a focus on Hsp90 and cancer. Expert Opin. Ther. Targets 10, 37–50 (2006).
Kamal, A., Boehm, M. F. & Burrows, F. J. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol. Med. 10, 283–290 (2004).
Neckers, L. & Neckers, K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics — an update. Expert Opin. Emerg. Drugs 10, 137–149 (2005).
Workman, P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 206, 149–157 (2004).
Zhang, H. & Burrows, F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J. Mol. Med. 82, 488–499 (2004).
Luo, W. et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc. Natl Acad. Sci. USA 104, 9511–9516 (2007).
Muchowski, P. J. & Wacker, J. L. Modulation of neurodegeneration by molecular chaperones. Nature Rev. Neurosci. 6, 11–22 (2005).
Geller, R., Vignuzzi, M., Andino, R. & Frydman, J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 21, 195–205 (2007).
Chiosis, G. & Neckers, L. Tumor selectivity of Hsp90 inhibitors: the explanation remains elusive. ACS Chem. Biol. 1, 279–284 (2006).
Duvvuri, M., Konkar, S., Hong, K. H., Blagg, B. S. & Krise, J. P. A new approach for enhancing differential selectivity of drugs to cancer cells. ACS Chem. Biol. 1, 309–315 (2006).
Kamal, A. et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425, 407–410 (2003).
Borkovich, K. A., Farrelly, F. W., Finkelstein, D. B., Taulien, J. & Lindquist, S. Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 9, 3919–3930 (1989).
Xu, W. et al. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl Acad. Sci. USA 99, 12847–12852 (2002).
Ali, M. M. et al. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006).
Bagatell, R. & Whitesell, L. Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol. Cancer Ther. 3, 1021–1030 (2004).
Whitesell, L. & Lindquist, S. L. HSP90 and the chaperoning of cancer. Nature Rev. Cancer 5, 761–772 (2005).
Mosser, D. D. & Morimoto, R. I. Molecular chaperones and the stress of oncogenesis. Oncogene 23, 2907–2918 (2004).
Takayama, S., Reed, J. C. & Homma, S. Heat-shock proteins as regulators of apoptosis. Oncogene 22, 9041–9047 (2003).
Falsone, S. F., Leptihn, S., Osterauer, A., Haslbeck, M. & Buchner, J. Oncogenic mutations reduce the stability of SRC kinase. J. Mol. Biol. 344, 281–291 (2004).
Xu, Y. & Lindquist, S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc. Natl Acad. Sci. USA 90, 7074–7078 (1993).
Xu, Y., Singer, M. A. & Lindquist, S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl Acad. Sci. USA 96, 109–114 (1999).
Dai, C., Whitesell, L., Rogers, A. B. & Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130, 1005–1018 (2007).
Acknowledgements
The author thanks L. Whitesell for invaluable comments on the manuscript. Work in the laboratory of L.E.C. is supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund and by a Canada Research Chair in Microbial Genomics and Infectious Disease.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Persister cell
-
A metabolically quiescent cell that neither grows nor dies when exposed to cidal concentrations of antimicrobial compounds.
- Polyene
-
A class of antifungal drug that intercalates into ergosterol-containing fungal membranes, thereby forming membrane-spanning channels that lead to the leakage of cellular components and cell death.
- Azole
-
A class of antifungal drug that inhibits fungal cytochrome P45014DM (also known as lanosterol 14α-demethylase), which is encoded by ERG11 and catalyses a late step in the biosynthesis of ergosterol; includes the triazoles (for example, fluconazole, voriconazole and posaconazole) and the imidazoles.
- Echinocandin
-
A class of antifungal drug that interferes with fungal cell-wall biosynthesis by inhibiting β-(1,3)-d-glucan synthase; includes caspofungin and micafungin.
- Major facilitator class
-
A large family of proteins that uses the energy that is provided by the proton motive force of the membrane to transport substrates across the membrane.
- ATP-binding cassette family
-
A member of a large family of proteins that uses the energy that is provided by the hydrolysis of ATP to transport substrates across membranes.
- Isochromosome
-
An abnormal chromosome that possesses a median centromere and two identical arms.
- Epigenetic variation
-
Variation that is caused by heritable changes that are not a result of a change in the DNA sequence.
- Immunophilin
-
A family of cis–trans peptidylprolyl isomerases that was originally studied as a cellular receptor for immunosuppressive drugs, such as cyclosporin A and FK506; includes cyclophilins and FK506-binding proteins.
Rights and permissions
About this article
Cite this article
Cowen, L. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol 6, 187–198 (2008). https://doi.org/10.1038/nrmicro1835
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1835
This article is cited by
-
Plastiphily is linked to generic virulence traits of important human pathogenic fungi
Communications Earth & Environment (2024)
-
Evaluation of the efficacy of heat shock protein inhibitors and antifungal drug combinations against Candida spp.
Rendiconti Lincei. Scienze Fisiche e Naturali (2023)
-
Strengthening collaborations at the Biology-Physics interface: trends in antimicrobial photodynamic therapy
Biophysical Reviews (2023)
-
Hsp90 and phosphorylation of the Slt2(Mpk1) MAP kinase activation loop are essential for catalytic, but not non-catalytic, Slt2-mediated transcription in yeast
Cell Stress and Chaperones (2022)
-
Cryptococcus spp. and Cryptococcosis: focusing on the infection in Brazil
Brazilian Journal of Microbiology (2022)