Key Points
-
Rickettsia spp. are Gram-negative, obligate, intracellular bacterial pathogens. Most rickettsial species have a life cycle that involves both an arthropod vector and a vertebrate host.
-
The high morbidity and mortality, ease of production and dissemination, and potential for aerosol spread are why both Rickettsia prowazekii and Rickettsia rickettsii category B and C are listed as potential bioterrorism agents.
-
R. prowazekii establishes latency in the infected host. Reactivation of infection causes Brill–Zinsser disease, a mechanism that could account for subsequent epidemics of louse-borne typhus.
-
Rickettsia spp. mainly infect endothelial cells, causing injuries that significantly contribute to the pathophysiological and clinical features of rickettsial diseases.
-
Spotted-fever-group rickettsiae subvert host cell signalling to avoid cell death and harness the actin machinery to enable intercellular spread.
-
Dendritic cells are an important factor in determining the type of acquired immune response and thus host susceptibility (or resistance) to rickettsial infection.
-
Tumour necrosis factor and interferon-γ are essential in vivo immune defence mechanisms that are directed against primary infection with Rickettsia spp. Crucial intracellular rickettsicidal mechanisms include nitric oxide, tryptophan degradation and reactive oxygen species.
-
Cytotoxic CD8+ T cells are crucial for establishing protective immunity against Rickettsia spp.
-
Comparative genomics of spotted-fever and typhus-group rickettsiae has enabled the identification of potential new virulence factors.
Abstract
Rickettsiae cause some of the most severe human infections, including epidemic typhus and Rocky Mountain spotted fever. Substantial progress has been made in research into the genomics, vector relationships, pathogenesis and immunity of these obligate, intracellular, arthropod-transmitted bacteria. This Review summarizes our understanding of the early and late events in pathogenesis and immunity, modulation of the host response to rickettsial infection by the vector, host defence, virulence mechanisms and rickettsial manipulation of host cells.
Similar content being viewed by others
Main
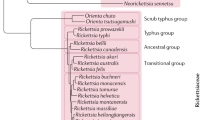
Pathogenic members of the Rickettsia genus are Gram-negative, obligate, intracellular bacteria that have a life cycle which involves both an arthropod vector and a vertebrate host1,2,3 (Fig. 1). Rickettsiae are classified into four groups based on their biological, genetic and antigenic characteristics4: the spotted fever group (SFG), typhus group, transitional group and ancestral group. SFG rickettsiae include highly pathogenic organisms, such as tick-transmitted Rickettsia rickettsii (Rocky Mountain spotted fever (RMSF))5,6, Rickettsia conorii (Mediterranean spotted fever)1,2,7, Rickettsia africae (African tick-bite fever)8,9, Rickettsia parkeri (mild-to-moderate spotted fever rickettsiosis, found in North and South America)10,11, Rickettsia slovaca (tick-borne lymphadenopathy)12,13, Rickettsia sibirica (North Asian tick typhus and lymphangitis-associated rickettsiosis)14, Rickettsia honei (found in Australia and Southeast Asia)15, Rickettsia japonica (found in Japan and Korea)16,17 and the apparently harmless Rickettsia montanensis, Rickettsia peacockii and Rickettsia rhipicephali2,18,19. The typhus group includes the highly pathogenic Rickettsia prowazekii (epidemic typhus) and Rickettsia typhi (murine typhus)20,21,22,23. The ancestral group includes Rickettsia bellii 24 and Rickettsia canadensis 25; whether these species are pathogens is unknown. The transitional group comprises Rickettsia akari (rickettsialpox)26, Rickettsia australis (Queensland tick typhus)27 and Rickettsia felis (flea-borne spotted fever)28 (Table 1). Rickettsia phylogeny has been addressed by sequence analyses of different genes, varying from housekeeping genes, which are useful for distinguishing distinct strains, to genes that are under evolutionary pressure, such as those that encode variable immunodominant outer-membrane proteins. This phylogenetic analysis has substantially affected the proposed taxonomy of rickettsiae. However, rickettsial taxonomy remains a controversial subject, owing to the absence of a universal consensus on those criteria that should be used for the designation of species (Box 1).
Rickettsiosis can present with an array of clinical signs and symptoms6,7,8,9,11,12,13,14,15,16,29,30,31. Highly lethal RMSF29,30,32,33 is characterized by headache, fever, myalgia, nausea and vomiting early in the illness; however, if untreated, severe injury can develop that sometimes progress to multi-organ failure. Systemic vascular infection in RMSF results in encephalitis, which leads to stupor, coma and seizures, interstitial pneumonia, non-cardiogenic pulmonary oedema and adult respiratory distress syndrome. In severe cases, hypovolaemia and hypotensive shock result in acute renal failure. Infection of a network of endothelial cells at the site of tick or mite inoculation of most SFG rickettsiae is followed by local dermal and epidermal necrosis that forms an eschar31. Disseminated infection, further injury to the vascular endothelium and infiltration of perivascular mononuclear cells leads to vasodilation, an increase in fluid leakage into the interstitial space and a characteristic rash. Epidemic typhus, which moulded world history for five centuries, is characterized by fever, headaches, mental confusion and a rash22,34 (Box 2). Similar to RMSF, epidemic typhus can develop into life-threatening conditions in previously healthy, immunocompetent individuals, unless they are treated early with an appropriate antibiotic. However, unlike RMSF, R. prowazekii causes latent infection in convalescent individuals, and recrudescence of latent R. prowazekii infection results in Brill–Zinsser disease, which is characterized by fever, rash and less-severe illness that nevertheless can infect feeding lice and ignite an epidemic35,36.
Interest in the pathogenesis of R. prowazekii and R. rickettsii has increased following their classification as select agents and as category B and C agents of potential bioterrorism, respectively, by the United States Centers for Disease Control and Prevention37 (see Further information). These pathogens are highly infectious agents that are easily disseminated and cause high morbidity and fatal disease, and thus require specific improvement in diagnostic tests and disease surveillance1,6 — the use of R. prowazekii as a biological weapon was initiated by the Soviet Union in the 1930s and Japan during the Second World War21,38.
The body of knowledge of rickettsial pathogenesis and immunity is based on disseminated infection of endothelial cells, the principal target host cells for rickettsiae. Human infections have rarely been investigated until the middle or late, and often fatal, stages of the illness. The best animal models for SFG rickettsioses use R. conorii and R. australis or typhus group rickettsiosis (using R. typhi) in susceptible mice that are inoculated intravenously: these manifest systemic endothelial-cell infection and characteristic pulmonary and cerebral lesions that recapitulate the clinical and pathological manifestations of the disease in humans. Infections of guinea pigs with R. rickettsii and R. prowazekii provide models of RMSF and epidemic typhus, respectively.
This Review highlights how the arthropod host acquires, maintains and transmits rickettsiae, the initial steps in pathogenesis and the subsequent interaction of the bacteria with cells in the endothelium, the main target cells. These events include: rickettsial entry, phagosomal escape, actin-based motility, cell-to-cell spread and the induction of cell injury. Regarding the host immune response to rickettsial infection, we will address innate and acquired immunity, with emphasis on recent data that illustrate the interaction of rickettsiae with dendritic cells (DCs). We also highlight some of the potential immunomodulatory effects of tick saliva on host defences and the immune response against Rickettsia spp.
Acquisition, interference and immunomodulation
Acquisition. Vertebrate hosts are infected with rickettsiae via direct inoculation by a feeding tick or mite or by scratching infected louse or flea faeces into their skin. Ticks with hard exoskeletal chitin are vectors and reservoirs for SFG rickettsiae. The principal vectors of RMSF in the United States are Dermacentor variabilis and Dermacentor andersoni (Table 1), which are most active during the late spring and summer, when RMSF peaks. Epidemic typhus (Box 2) caused by R. prowazekii is associated with cold weather and lack of hygiene22, and hasre-emerged in louse-infested populations. Humans in endemic regions, as well as the eastern flying squirrel Glaucomys volans volans20,39, and its flea and louse in the United States, and ticks in Mexico and Africa are the known reservoirs of R. prowazekii40,41.
Interference. Infection of a tick with one SFG rickettsial species seems to interfere with infection by a second SFG rickettsial species. It was suggested that rickettsial infection of tick ovaries might alter the molecular-expression profiles of the oocytes and cause interference or blocking of the second infection42,43. This process of rickettsial 'interference' might affect the frequency and distribution of different pathogenic rickettsiae, and could explain the limited distribution of virulent R. rickettsii in the eastern part of the Bitterroot Valley, Montana, USA, where they infect less than 1% of wood ticks42,44. The low infection rate of R. rickettsii is attributed to the high infection rate of female wood ticks (D. andersoni) in the eastern, but not western, Bitterroot Valley with non-virulent rickettsiae, particularly R. peacockii (70% in the eastern compared with 4% in the western side of Bitterroot Valley)19,44. In most geographic locations, fewer than 0.1% of Dermacentor spp. ticks carry R. rickettsii45. These data correspond to the focality of RMSF in the west side of the valley, where most human cases result from exposure to west-side ticks (D. andersoni). Unlike pathogenic R. rickettsii, which is lethal for ticks46 and highly virulent in guinea pigs47, infection with R. peacockii does not cause a reduction in tick viability, and might even be beneficial for tick hosts by antagonizing superinfection of ovarian tissues by R. rickettsii.
Ticks acquire SFG rickettsial species through transovarial transmission (adult female to egg) and trans-stadial passage (egg to larva to nymph to adult), and by horizontal acquisition during feeding on a rickettsemic host. Most SFG rickettsiae are probably maintained in nature by all these mechanisms (Fig. 1). Therefore, the adverse effect of virulent R. rickettsii on the viability of adult ticks and maintenance of Rickettsia spp. in nature are probably balanced by the feeding of susceptible ticks on a rickettsemic host, which functions as an amplifying reservoir for rickettsiae. In fact, it has been shown that despite the high mortality of experimentally infected ticks, many larvae that acquire rickettsiae during feeding survive and are capable of transmitting the infection as nymphs. This suggests that nymphs are a crucial link for R. rickettsii maintenance and transmission between vertebrates. Importantly, colonies of R. rickettsii-infected ticks have been observed to maintain the infection without overt deleterious effects for several generations. The pathological effect of rickettsiae on ticks would explain the occurrence of RMSF in endemic regions, despite the low prevalence of naturally infected adult ticks45,46.
Immune modulation. Studies of the virulence of rickettsiae within their tick vector revealed that feeding ticks or incubating them at 37°C for 24–48 hours before their inoculation onto non-immune guinea pigs results in severe disease, compared with asymptomatic infection following inoculation of guinea pigs with infected ticks that were maintained at 4°C or starved for a prolonged period48. This observation, described as the reactivation phenomenon by Parker and Spencer44, refers to changes in the virulence of rickettsiae that are linked to the physiological status of the ticks.
As an immune evasion or modulation mechanism that allows the ticks to feed for several days or weeks, ticks inoculate their saliva with anti-haemostatic components that are crucial for the enhancement of blood feeding and salivary immunomodulatory components that enhance pathogen transmission and prevent the host from rejecting the ticks49,50,51,52,53. For example, the saliva of ticks inhibits neutrophil function52, interferes with the complement system49,50,51, natural killer (NK) cell and macrophage activity54, decreases the production of cytokines, such as interleukin-12 (IL-12) and interferon-γ (IFN-γ), and decreases T-cell proliferation55,56. Tick-infested mice do not develop resistance to further infestations with Rhipicephalus sanguineus, and the immune response in infested mice exhibits a T helper 2 (TH2)-type pattern56,57. Tick saliva might influence T-cell-effector functions through its initial interaction with professional antigen-presenting cells, namely DCs58. Such initial interactions can subsequently influence the differentiation towards either a TH2-cell phenotype (an ineffective acquired immune response against intracellular pathogens such as Rickettsia spp.) or an immunosuppressive phenotype58,59. Indeed, the addition of tick saliva to bone-marrow-derived DCs inhibits their maturation by decreasing the expression of co-stimulatory (CD40, CD80 and CD86) and adhesion (CD54) molecules58,59. Furthermore, the maturation of DCs that is stimulated by lipopolysaccharide in the presence of tick saliva results in reduced expression of co-stimulatory molecules and reduced production of IL-12, but not immunosuppressive IL-10. More importantly, DCs cultured with tick saliva are inefficient in the induction and activation of antigen-specific, cytokine-producing T cells58,59. As discussed below, fully mature DCs are crucial for induction of an effective TH1 response against Rickettsia spp. Therefore, it is possible that suppression of DC maturation by tick saliva during the initial stages of rickettsial infection could interfere with their co-stimulatory and antigen-presentation functions. Such suppression of DC maturation would adversely influence the acquired immune response against tick-transmitted Rickettsia spp., thereby leading to increased host susceptibility to severe and fatal rickettsial disease. However, because the tick host is an important component in the life cycle of rickettsiae, further studies are required to address important questions related to vector biology and disease pathogenesis, such as whether tick saliva enhances Rickettsia spp. infectivity during natural transmission and whether pre-exposure to saliva from uninfected ticks that generates immunity to saliva protects the vertebrate host, particularly in endemic areas, from natural tick-transmitted rickettsial infection. If immunity to salivary components can protect against natural rickettsial infection, an effective anti-rickettsial vaccine could be designed that contains tick salivary proteins that act as an adjuvant with specific rickettsial antigens.
Rickettsia–endothelial cell interactions
Rickettsial entry. R. conorii OmpB binds specifically to Ku70 (Fig. 2), a component of the DNA-dependent protein kinase60. The binding and recruitment of Ku70 to the plasma membrane are important events in the entry of R. conorii into non-phagocytic mammalian cells60. Although nuclear Ku70 is translocated to the cytoplasm and plasma membrane, where it inhibits apoptosis and mediates homologous and heterologous cell adhesion and fibronectin binding, it has been proposed that the presence of Ku70 within lipid rafts might have an important role in the signal transduction that leads to induced phagocytosis. The role of cholesterol as an essential component of the membrane receptor that binds to R. prowazekii was described previously61. Similar to other intracellular pathogens, such as Listeria monocytogenes , the entry of R. conorii into non-phagocytic cells is dependent on membrane cholesterol. Ku70 is present within lipid microdomains that are enriched in lipid-raft components. The association of Ku70 with lipid microdomains and its binding to R. conorii suggest that Ku70 has an important role in cholesterol-dependent bacterial entry60. Although the exact mechanism by which Ku70 supports the entry of R. conorii into non-phagocytic cells remains unclear, the binding of R. conorii OmpB to Ku70 might activate membrane Ku70, which is postulated to lead to the activation of a cascade of signalling events, including the small GTPase, Cdc42, phosphoinositidyl-3-kinase, src-family tyrosine kinases and the tyrosine phosphorylation of focal adhesion kinase62. These signalling events are known to be strongly associated with β1-integrin activation and bacterial entry63 (Fig. 2). Similar to the entry of L. monocytogenes into its host cells, R. conorii infection stimulates the ubiquitination of Ku70. In addition, the ubiquitin ligase c-Cbl is recruited to R. conorii-entry foci, and downregulation of endogenous c-Cbl blocks bacterial invasion and Ku70 ubiquitination60,62. The binding of Ku70 to OmpB and the role of Ku70 in bacterial entry into host cells correlate with the decreased expression of OmpB that is associated with reduced virulence of R. rickettsii str. Iowa64 and the observation that anti-OmpB antibodies protect animals from an otherwise lethal challenge of R. conorii65,66,67. However, it is possible that SFG rickettsial OmpA or other unidentified rickettsial outer-membrane proteins also mediate adhesion by binding to unknown receptors68.
a | Spotted-fever-group rickettsiae attach to Ku70 on the surface of human target cells (the endothelium) via outer-membrane-protein B (OmpB) and to an unknown receptor via outer-membrane-protein A. b | Cbl ubiquitinates Ku70 (Ref. 61), and signal-transduction events that involve Cdc42, protein tyrosine kinase, phosphatidylinositol 3′-kinase (PI3-K) and Src-family kinases activate the Arp 2/3 complex to induce cytoskeletal actin to phagocytose the rickettsia62. c | Membranolytic phospholipase D and haemolysin C mediate rickettsial phagosomal escape83. d | RickA-stimulated activation of Arp2/3-mediated polymerization of host actin propels the bacterium through the cytosol and into filopodia. e | Rickettsiae are then either released from filopodia extracellularly or spread into the adjacent cell84,85,86,87,88. Cbl, family of ubiquitin ligases; N-WASP, neural Wiskott–Aldrich syndrome protein; Ub, ubiquitin.
Rickettsial diseases and endothelial pathogenesis. Most of the clinical characteristics of rickettsial diseases are attributed to disseminated infection of the endothelium, where they grow and stimulate oxidative stress, thereby causing injury to the endothelial cells. Severe morbidity and mortality of RMSF are due to effects such as cerebral oedema and non-cardiogenic pulmonary oedema. The most prominent pathophysiological effects of rickettsial infection of endothelial cells include: an increase in vascular permeability; generalized vascular inflammation; oedema; increased leukocyte–endothelium interactions; and release of powerful vasoactive mediators that promote coagulation and pro-inflammatory cytokines69,70. Evidence that supports a pro-coagulant and pro-inflammatory phenotype of the host response is provided by studies of cultured endothelial cells in which rickettsial infection causes increased expression of tissue factor, thrombomodulin plasminogen-activator inhibitor 1, IL-1, IL-6, IL-8 and E-selectin70,71,72,73. Increased plasma levels of von Willebrand factor that are associated with increased levels of inflammatory cytokines, such as IL-6, have also been detected in patients with African tick-bite fever and Mediterranean spotted fever69,74. Prostaglandins and leukotrienes are crucial vasoactive modulators of vascular tone and permeability that are potential mediators of microvascular injury and vasculitis in rickettsial infection75,76. These vasoactive substances are generally generated by an inducible isoenzyme cyclooxygenase (COX)75. Transcriptional activation of host endothelial cells in response to stimulation with R. rickettsii or R. conorii involves rapid regulation of COX2 expression and inhibition of COX2 activity during infection, which leads to decreased levels of secreted prostaglandins. As a regulatory mechanism that prevents the development of extensive vascular injury, endothelial cells that are infected with R. rickettsii produce haem oxygenase, an antioxidant, anti-inflammatory and vasoprotective enzyme that controls COX2 activity77. The production of this antioxidant mechanism by R. rickettsii-infected cells seems to be dependent on several factors, including: dose and kinetics of rickettsial infection; viability of the host cells; de novo protein synthesis by host cells; adhesion and entry of Rickettsia spp. to the host cell membrane; rickettsial replication; and viability77. Viability is probably influenced by the host immune status, as well as whether patients are treated with doxycycline at an early stage of infection. Nevertheless, the balance between the production of vasoprotective, anti-inflammatory and antioxidant haem oxygenase and the generation of vasoactive substances by COX2 in Rickettsia-infected endothelial cells might determine the outcome of rickettsial infection and thus the susceptibility or resistance to severe, and sometimes fatal, disease.
In an in vitro model of human microvascular endothelium, R. rickettsii causes early-dose-dependent increased vascular permeability. Furthermore, pro-inflammatory cytokines, such as IL-1β and tumour necrosis factor (TNF), which are probably produced in vivo by perivascular T lymphocytes and macrophages, cause a further enhancement of vascular permeability that is not dependent on nitric oxide production78. Increased permeability of endothelial monolayers that are infected with R. rickettsii in the presence of pro-inflammatory cytokines is associated with disruption of intercellular adherens junctions and redistribution of p120 and β-catenin proteins — these proteins attach the endothelial cells to the extracellular matrix and regulate the functional interaction of vascular endothelial (VE)–cadherin with the actin cytoskeleton — from the cell–cell junction by a nitric-oxide-independent mechanism. The cellular and molecular mechanism by which rickettsiae directly increase endothelial permeability is not yet clear. R. rickettsii-infected human-derived microvascular endothelial cells produce nitric oxide, which has been shown to play a part in increasing vascular permeability during rickettsial infection. However, blocking nitric oxide production does not influence endothelial-cell monolayer integrity despite an increase in the number of intracellular rickettsiae. These data suggest that other downstream or upstream intracellular molecules produced by endothelial cells at early stages after infection cause increased vascular permeability. Possible effectors could include reactive oxygen species or vascular endothelial growth factor, which have been associated with vascular dysfunction in endothelial-cell infections that are caused by other pathogens.
Rickettsiae inhibit endothelial-cell apoptosis by a mechanism that involves nuclear factor-κB (NF-κB) activation79,80, an immune evasion strategy that enables rickettsiae to survive and replicate within the endothelium. NF-κB is a transcription factor that regulates many inflammatory genes that are involved in cytokine and chemokine production. The anti-apoptotic effect of rickettsiae on endothelial cells is linked to the pro-inflammatory phenotype of those cells. Lymphocyte adhesion to the endothelium is a crucial step for transmigration of lymphocytes to the areas of inflammation that are beneath the endothelium. The production of pro-inflammatory cytokines, such as IL-1α, IL-6 and IL-8, by endothelial cells promotes the expression of endothelial-cell adhesion molecules, such as intercellular-adhesion molecule 1 and vascular-cell-adhesion molecule 1, which support the recruitment of T cells to the site of infection. Rickettsiae also enhance the expression of chemokine receptors, such as CXC-chemokine receptor 3, and the production of chemokines, such as CXC-chemokine ligand 9 (CXCL9; also known as MIG) and CXCL10 (also known as IP10), by infected endothelial cells81,82 (Fig. 3). The peak of expression of these chemokines correlates with maximal T-cell infiltration (mainly CD8+ T cells) at the site of infection82. However, it is not yet clear whether greater chemokine production and increased T-cell migration to the site of infection contribute more to the pathogenesis of severe disease or to protection against rickettsial infection.
This hypothetical model is based on in vitro and in vivo studies in an animal model of mild disseminated spotted-fever rickettsiosis that was caused by sublethal rickettsial infection. Immature dendritic cells (DCs) that encounter rickettsiae in the peripheral tissues, such as skin and lung, undergo maturation and migrate to secondary lymphoid tissues (for example, draining lymph nodes), where they activate antigen-specific T lymphocytes. Rickettsial infection stimulates DC maturation (upregulation of the major histocompatibility complex and co-stimulatory molecules CD80, CD86 and CD40). Interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), which are produced by other cells of the innate immune system, such as natural killer (NK) cells or infected endothelial cells, could promote complete DC maturation, which is marked by the production of interleukin-12 (IL-12). IL-12 activates NK cells, which are crucial for the early clearance of rickettsiae. Mature Rickettsia-infected DCs enter lymph nodes through afferent lymphatic vessels, where they display antigens to naive antigen-specific CD4+ and CD8+ T cells and provide co-stimulatory signals that activate antigen-specific T cells. Activated antigen-specific CD4+ and CD8+ T cells proliferate and differentiate into antigen-specific effector-type-1 cells that produce IFN-γ and TNF-α. Induction of TH1 cells is mediated, in part, by IL-12 production by DCs. In addition, antigen-specific B cells proliferate and differentiate into antibody-secreting cells. Rickettsia-specific antibodies have an important role in protection against re-infection. These differentiated B and T lymphocytes migrate back to the blood and then to the site of infection, where they mediate the clearance of rickettsiae. Production of chemokines, such as CXC-chemokine ligand 9 (CXCL9) and CXCL10, by infected endothelial cells enhances lymphocyte migration. Production of IFN-γ and TNF-α by CD4+ TH1 effectors and CD8+ cytotoxic T lymphocytes (CTLs) activates infected target cells, such as DCs, macrophages and endothelial cells. Activated infected cells kill rickettsiae by producing intracellular rickettsicidal molecules; for example, nitric oxide. Further clearance of rickettsial infection requires cytotoxic antigen-specific CD8+ T cells that eliminate Rickettsia-infected cells by a perforin-mediated mechanism.
Actin-dependent movement of rickettsiae. The entry of both SFG and typhus-group rickettsiae into mammalian cells is a dynamic process between metabolically active Rickettsia spp. and mammalian cells. Typhus-group rickettsiae enter the endothelial cells through adherence to undefined receptors and induced phagocytosis, followed by escape to the cytosol, which is mediated by phospholipase D and haemolysin C83. Replication of R. prowazekii inside host cells is followed by the rupture of infected cells and release of rickettsiae, which infect neighbouring cells. Spotted-fever rickettsiae can manipulate host cell signalling and endocytic pathways to their advantage, similar to L. monocytogenes84. SFG Rickettsia spp. harness the actin-polymerization machinery in the cytoplasm to facilitate intracellular and intercellular movement. After invasion of the host and entry into the cytosol, most SFG rickettsiae are propelled by polymerization of the host cell cytoskeletal protein actin at the surface of one pole of the rickettsiae. Unlike Listeria spp. and Shigella spp., which also escape into the cytosol, the actin tails of Rickettsia spp. are much less dense and comprise several distinct bundles of long, unbranched filaments that are similar to those present in filopodia85,86. The spread of Rickettsia spp. from cell to cell enables rickettsiae to avoid contact with immune-cells and antibodies, which could be a possible immune-evasion mechanism. A comparison of the complete genome sequence of R. conorii with that of typhus group R. prowazekii, which does not have actin tails and thus does not manifest an actin-based motility85, identified a 2 kb R. conorii-specific region that encodes a predicted protein of 517 amino acids, known as RickA86. RickA contains a central proline-rich domain and a carboxy-terminal WH2 (Wiskott–Aldrich syndrome protein (WASP) homology 2) domain87. WH2 binds to actin monomers, which is followed by binding to a region with homology to the central and acidic domain of WASP-family proteins, including an amphipathic helix that is predicted to bind the Arp2/3 complex87,88 (Fig. 2). Activation of Arp2/3 then results in nucleation of actin polymerization and induction of the formation of a network of long, unbranched filaments in Rickettsia spp. actin tails87. RickA proteins from R. conorii and R. rickettsii have a single WH2 domain that is similar to WASP, whereas R. montanensis RickA has two WH2 domains that are similar to neural-WASP. The exact role of RickA in intracellular motility, cell-to-cell spread and virulence of SFG rickettsiae has not been completely determined owing to a lack of genetic tools. However, lack of motility and virulence in R. peacockii, an SFG rickettsia that has a naturally occurring transposon insertion in rickA, indicates a possible role for RickA in the motility (and perhaps virulence) of SFG rickettsiae89 (Table 2).
Host response to infection
Innate immunity. Early resistance to infection is attributed to the production of IFN-γ by NK cells and the resultant activation of infected target cells, principally endothelial cells, DCs and macrophages (Fig. 3). IFN-γ and TNF are essential for primary defence against infection with Rickettsia spp., and mice that lack these cytokines develop an overwhelming infection and succumb to an ordinarily sublethal dose of rickettsiae90,91. Eschar lesions from patients with mild-to-moderate boutonneuse fever express high mRNA levels of TNF, IFN-γ, IL-10, RANTES, indoleamine-2,3-dioxygenase (an enzyme that is involved in limiting rickettsial growth by tryptophan degradation) and inducible nitric oxide synthase (a source of microbicidal nitric oxide)92. Significantly high levels of intralesional IL-10 are inversely correlated with low levels of IFN-γ and TNF. Skin lesions from patients with severe BF express higher levels of RANTES than those with mild or moderate disease92. However, it is not clear whether these cytokine and chemokine responses in patients with BF are simply a correlate of mild and severe disease or contribute to anti-rickettsial immunity and pathogenesis.
At the cellular level, cytokine-activated endothelial cells and macrophages are the principal mediators of the killing of rickettsiae, as they are also the major target cells. Although the mechanisms of bacterial killing by recruited lymphocytes and inflammatory cells in vivo are not completely defined, the induction of nitric oxide (Fig. 3), oxidative burst, production of hydrogen peroxide and/or tryptophan degradation by endothelial cells and macrophages contribute to pathogen clearance in vitro93.
Interaction of rickettsiae with dendritic cells. Although extensive studies have examined the acquired immune response against Rickettsia spp., there is still a large gap in our understanding of the initial interaction between tick-transmitted rickettsiae and host immune cells at the site of inoculation (the dermis of the skin) and the effect of this interaction on the acquired immune response in secondary lymphoid organs. Transmission of rickettsiae through the dermis suggests that resident DCs might have an important role in innate and acquired immunity. Rickettsia spp. effectively infect bone-marrow-derived DCs (BMDCs). Compared with their immediate and exclusive cytoplasmic localization within endothelial cells, rickettsiae efficiently enter and localize in both phagosomes and the cytosol of BMDCs94. The dual intracellular localization of rickettsiae within BMDCs favours the access of rickettsial antigen to both major histocompatibility complex (MHC) class I and II pathways, thus promoting the activation of Rickettsia-specific CD8+ and CD4+ T cells, respectively. Rickettsiae have been shown to induce the maturation of BMDCs, which results in the upregulation of CD40, CD80, CD86 and MHC-II and production of IL-2, IL-12p40 and IL-23 — IL-12p40 and IL-23 are prototypic TH1-promoting cytokines94. DCs efficiently present rickettsial antigens to T cells, as shown by the ability of R. conorii-infected DCs to effectively activate immune CD4+ and CD8+ T cells that were derived from R. conorii-infected mice and stimulate the TH1 response owing to the production of substantial quantities of IFN-γ94,95 (Fig. 3). Interestingly, Rickettsia-infected DCs activate naive CD8+ T lymphocytes in vitro in the absence of CD4+ T-cell help95 (Fig. 3) together with the induction of DC maturation by rickettsiae. Thus, Rickettsia-infected DCs provide signal 1 (TCR stimulation) and signal 2 (IL-2 and TH1 cytokines) to naive T cells, which accounts for their ability to induce activation and differentiation of T cells (Fig. 3). Interestingly, the transfer of rickettsiae-stimulated DCs protects mice from lethal rickettsial challenge by limiting rickettsial proliferation in vivo, whereas partial protection is observed in mice that receive LPS-stimulated DCs95. Protection against R. conorii following the transfer of DCs is associated with the production of antigen-specific IFN-γ by T cells and the expansion of NK cells.
The role of DCs in resistance and susceptibility to fatal SFG rickettsiosis has been investigated in susceptible C3H/HeN mice and highly resistant C57BL/6 mice, which remain healthy after a dose of R. conorii that kills 100% of C3H/HeN mice94. Early effective bacterial elimination in C57BL/6 mice suggests that the crucial immune effectors that determine the resistance or susceptibility to fatal disease are determined by innate immune cells. BMDCs of resistant mice internalize greater quantities of rickettsiae, kill rickettsiae more effectively, express higher levels of MHC-II, produce more IL-12p40 and are more potent in priming naive IFN-γ to produce CD4+ TH1 cells. By contrast, BMDCs of susceptible mice fail to induce CD4+ T-cell activation and differentiation into the TH1 or TH2 phenotype in an in vitro co-culture system. The CD4+ T-cell suppressive function of Rickettsia-infected BMDCs from susceptible mice is associated with the expansion of forkhead box P3 (Foxp3)+CD4+ T-regulatory cells in an in vitro co-culture system94 (Fig. 4). These data suggest that rickettsiae stimulate DCs to develop a protective TH1 response in resistant hosts but induce suppressive adaptive immunity in susceptible hosts, which is probably mediated by infection-induced T-regulatory cells94. Our preliminary data suggest that a unique subset of inducible T-regulatory cells play a crucial part in mediating fatal disease in an animal model of disseminated, lethal SFG rickettsiosis. We are currently undertaking several approaches to identify the immune regulatory mechanism (or mechanisms) by which T-regulatory cells mediate immune suppression in fatal SFG rickettsiosis. Taken together, studies of the early interaction between SFG Rickettsia spp. and DCs not only enhance our understanding of the cellular immune mechanisms that account for mild and severe rickettsial disease, but can also be used to help develop effective and optimal immunotherapeutic approaches that both enhance protective immunity and abrogate the immunosuppressive mechanisms that are responsible for fatal spotted-fever rickettsial diseases.
Rickettsial infection stimulates partial DC maturation, as shown by the upregulation of major histocompatibility complex (MHC) and co-stimulatory molecules. Bone-marrow-derived DCs from susceptible murine hosts that are infected in vitro with Rickettsia fail to stimulate Rickettsia-specific CD4+ T-cell differentiation into TH1 or TH2 cells that produce interferon-γ (IFN-γ) or interleukin (IL)-4, respectively. Suppressed effects on CD4+ T-cell responses are associated with IL-10 production by DCs as well as increased numbers of CD4+CD25+Foxp3− T-regulatory (TReg) cells that also secrete IL-10. This process suggests that immune suppression is a mechanism that contributes to the development of progressive, fatal spotted-fever rickettsiosis.
NK cells provide an early source of IFN-γ in immune responses to species of Rickettsia (Fig. 3), with a significant expansion of NK cells occurring 2 days after rickettsial infection. Depletion of NK cells enhances the susceptibility of mice to R. conorii infection96. Enhanced NK activity during rickettsial infection is associated with high serum levels of IL-12 and IFN-γ (Fig. 3), which suggests that NK cells have a role in mediating the protective TH1 response in anti-rickettsial immunity96. Current studies in our laboratory are directed towards analysing early DC–NK crosstalk in vitro and in vivo following sublethal and lethal rickettsial disease in the presence or absence of tick saliva. These studies could provide essential information that is necessary for molecular identification of rickettsial ligands or vaccine candidates that stimulate innate immune cells and protective specific immunity against Rickettsia spp.
Acquired immune response against Rickettsia spp. Animal models of disseminated rickettsiosis caused by intravenous R. conorii or R. australis infection in C3H/HeN or C57BL/6 mice, respectively, have revealed the crucial roles of T- and B-cell responses against rickettsiae (Fig. 3). Experiments with mice that were depleted of, or deficient in, particular subsets of cells or cytokines90,91,97 demonstrated that a deficiency of CD8+ T cells, IFN-γ or TNF markedly increases the susceptibility of mice to infection with R. conorii. CD8+ T cells mediate their effector function against Rickettsia spp. through both IFN-γ production and cytotoxic killing of infected target cells. In humans, perivascular infiltrates of CD8+ and CD4+T cells are present in skin lesions of patients with SFG rickettsiosis98. Depletion and adoptive-transfer experiments indicate that CD4+ TH1 cells mediate protective immunity against Rickettsia spp. through several pathways: the production of IFN-γ and TNF, which activate microbicidal activities of infected target cells; Rickettsia-specific CD4+T-cell help to antigen-specific B cells and therefore antibody production, an important host defence against re-infection; and CD4+ T-cell help for the induction of cytotoxic CD8+ T cells, an indispensable component of protective cellular immunity against species of Rickettsia (Fig. 3). Polyclonal antibodies to R. conorii and monoclonal antibodies to OmpA and OmpB provide effective passive immunity in infected severe combined immunodeficient mice66. This antibody-mediated protection is Fc-dependent, suggesting that antibodies have a role in opsonization and phagocytosis of extracellular rickettsiae. Interestingly, polyclonal antibodies and an anti-OmpB monoclonal antibody inhibit the escape of R. conorii from the phagosome of endothelial cells or macrophages, which results in phagolysosomal killing through nitric oxide, reactive oxygen intermediates and L-tryptophan starvation99. These observations suggest that anti-rickettsial antibodies, including anti-OmpB, neutralize an antigenic determinant on a rickettsial protein that plays a part in the escape of rickettsiae to the cytosol. Similar to SFG rickettsiae, the immune reactivity and immunogenicity of R. typhi and R. prowazekii OmpB have been characterized in an attempt to identify synthetic antigens that can be used in specific diagnostic immunoassays or candidate vaccines. These studies show that R. typhi and R. prowazekii OmpB are indeed immune reactive and immunogenic as measured by the ability of purified OmpB from R. typhi or R. prowazekii (Madrid E and Breinl strains, respectively) to induce strong proliferation and IFN-γ production in vitro through human CD4+ T-cell clones from individuals who are seropositive for R. typhi100 and the ability of purified OmpB of R. typhi to stimulate humoral and cell-mediated immune responses in guinea pigs and mice101,102. The antibody-binding sites and linear peptide epitopes on these proteins were characterized102. However, the limited numbers and weak immune reactivity of these linear peptide epitopes against patient sera suggest that they are not useful for diagnosis. Yet these studies do not exclude the possibility that conformational epitopes of OmpB or other outer-membrane or cytoplasmic proteins of SFG or typhus-group rickettsiae play an important part in protection. In support of this possibility is the recent finding that immunization with avirulent R. rickettsii str. Iowa that is deficient in OmpA and defective in the processing of OmpB from the 168 kDa precursor form to the 135 and 32 kDa forms of the protein protects 90% of guinea pigs against disease that is caused by virulent R. rickettsii str. Sheila Smith103. Interestingly, lack of protection in one of the vaccinated animals was associated with defective antibody production, which is consistent with studies which indicate that antibodies of an as-yet-unidentified antigen specificity or isotype have a role in protection against Rickettsia spp. Interesting questions include whether T-cell mediated immunity is responsible in large part for protection in these animals and which immunodominant or subdominant rickettsial antigens or peptides are recognized by B and T cells in protected animals that are vaccinated with avirulent R. rickettsii str. Iowa. That OmpA, OmpB and the unrelated T-cell-responsive antigens are crucial stimulators of immunity has been clearly established104,105,106.
Conclusions and future directions
Analysis of rickettsial genome sequences (Box 3) and investigation of rickettsiae–host cell interactions have identified rickettsial adhesins, a host cell receptor, components of signal transduction that affect rickettsial entry and apparent mediators of phagosomal escape and manipulation of host cell function, such as the activation of NF-κB to inhibit apoptosis, actin-based motility and cell-to-cell spread. However, most of these studies were exploratory rather than mechanistic, which is confounded by the problem of in vitro studies in which an interaction between Rickettsia spp. and one particular host cell has often been studied without considering the complex host–microbial interaction that occurs in vivo. In addition, the lack of an effective genetic system for rickettsiae hinders the identification of virulence genes and elucidation of their effect on the host. Thus, although rickettsial research has generated a wealth of data, we still lack crucial information that is directly relevant to human disease and can be effectively applied towards vaccines and immunotherapy.
Examples of the gaps in our knowledge include: the crucial rickettsial virulence factor (or factors) that mediates immune evasion or modulation and intracellular survival; the immunodominant and subdominant T-cell epitopes that mediate protective immunity against species of Rickettsia; and the functions of rickettsial genes that have been annotated without experimental analysis. The exact pathophysiological and immune mechanism (or mechanisms) that accounts for host resistance or susceptibility to virulent Rickettsia species, such as R. rickettsii and R. conorii, or accounts for mild disease following infection with less-virulent rickettsial species; the microbial or host-related regulatory mechanisms that abrogate or enhance tissue injury and progression of the disease; and the mechanisms that control the induction of these regulatory mechanisms also remain unknown. Furthermore, the effect of variables such as infectious dose, route of infection and host genetic susceptibility on host defence against Rickettsia species; the immunomodulatory effects of tick saliva at the site of inoculation; the course and route of rickettsial spread from the skin; the mechanism and importance of rickettsial-infection-associated immunosuppression; and the mechanism of perivascular emigration of immune CD4+ and CD8+ T lymphocytes and macrophages at the site of microvascular infection require further elucidation.
More importantly, what is the relevance of these in vitro studies and in vivo animal studies to humans, taking into consideration important factors such as age, gender, genetic polymorphisms, immune status, pre-existing morbidity and risk factors, such as diabetes and alcoholism. Future progress is required to identify the complete set of rickettsial adhesins and host cell receptors, the importance of Rickettsia-induced oxidative stress on endothelial cells in vivo and other mechanisms of cell and tissue injury in rickettsial diseases. A reliable genetic system is needed for inactivation of rickettsial genes to attribute functions to putative virulence factors, including phospholipase D, haemolysin C and RickA (Table 2). The contributions of cytokines, cytotoxic T lymphocytes and T-regulatory cells to the pathogenesis of tissue injury in rickettsial infection also need to be further determined. To understand the mechanisms of the most important pathophysiological mechanisms in rickettsioses, the relative roles of oxidative stress, pro-inflammatory cytokines, vascular endothelial growth factor and other factors should be analysed. For vaccine development, it is important to identify the immunodominant rickettsial antigens that stimulate CD4+ and CD8+ T lymphocytes to secrete protective cytokines, stimulate protective antibodies, in addition to anti-OmpA and anti-OmpB, and stimulate cytotoxic CD8+ T lymphocytes that eliminate rickettsiae-infected endothelial cells.
References
Walker, D. H. Rickettsiae and rickettsial infections: the current state of knowledge. Clin. Infect. Dis. 45 (Suppl. 1), 39–44 (2007).
Yu, X. J. & Walker, D. H. in Bergey's Manual of Systematic Bacteriology 2nd edn Vol. 2 (eds Brenner, D. J., Kreig, N. R. & Staley, J. T.) 96–116 (Springer, New York, 2005).
Azad, A. F. & Beard, C. B. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 4, 179–186 (1998).
Gillespie, J. J. et al. Plasmids and rickettsial evolution: insight from Rickettsia felis. PloS One 2, e266 (2007).
Ricketts, H. T. The study of “Rocky Mountain spotted fever” (tick fever?) by means of animal inoculations. JAMA 47, 33–36 (1906).
Walker, D. H. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin. Microbiol. Rev. 2, 227–240 (1989).
Parola, P., Paddock, C. D. & Raoult, D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin. Microbiol. Rev. 4, 719–756 (2005).
Jensenius, M., Fournier, P. E., Kelly, P., Myrvang, B. & Raoult, D. African tick bite fever. Lancet Infect. Dis. 3, 557–564 (2003).
Raoult, D. et al. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 344, 1504–1510 (2001).
Parker, R. R. in Proc. 3rd Int. Congr. Microbiol. 390–391 (1940).
Paddock, C. D. et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38, 805–811 (2004).
Lakos, A. Tick-borne lymphadenopathy — a new rickettsial disease? Lancet 350, 1006–1008 (1997).
Oteo, J. A. et al. Dermacentor-borne necrosis erythema and lymphadenopathy: clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 10, 327–331 (2004).
Fournier, P. E., Gouriet, F., Brouqui, P., Lucht, F. & Raoult, D. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: seven new cases and review of the literature. Clin. Infect. Dis. 40, 1435–1444 (2005).
Stenos, J., Roux, V., Walker, D. H. & Raoult, D. Rickettsia honei sp. nov., the aetiologic agent of Flinders Island spotted fever in Australia. Int. J. Syst. Bacteriol. 48, 1399–1404 (1998).
Uchida, T., Uchiyama, T., Kumano, K. & Walker, D. H. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int. J. Syst. Bacteriol. 42, 303–305 (1992).
Mahara, F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg. Infect. Dis. 3, 105–111 (1997).
Burgdorfer, W. et al. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect. Immun. 12, 205–210 (1975).
Niebylski, M. L. et al. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int. J. Syst. Bacteriol. 47, 446–452 (1997).
Bozeman, F. M., Masiello, S. A., Williams, M. S. & Elisberg, B. L. Epidemic typhus rickettsiae isolated from flying squirrels. Nature 255, 545–547 (1975).
Azad, A. F. Pathogenic rickettsiae as bioterrorism agents. Clin. Infect. Dis. 45 (Suppl. 1), 52–55 (2007).
Raoult, D. et al. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 352, 353–358 (1998).
McLeod, M. P. et al. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J. Bacteriol. 186, 5842–5855 (2004). An annotation of the genome of R. typhi that built on the previously sequenced Rickettsia spp. genomes to provide a picture of Rickettsia functional diversity.
Ogata, H. et al. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet. 2, e76 (2006).
McKiel, Y. A., Bell, E. J. & Lackman, D. B. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Can. J. Microbiol. 13, 503–510 (1967).
Paddock, C. D. et al. Isolation of Rickettsia akari from eschars of patients with rickettsialpox. Am. J. Trop. Med. Hyg. 75, 732–738 (2006).
Andrew, R., Bonin, J. M. & Williams, S. Tick typhus in North Queensland. Med. J. Aust. 2, 253–258 (1946).
Bouyer, D. H. et al. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int. J. Syst. Evol. Microbiol. 51, 339–347 (2001).
Dumler, J. S. & Walker, D. H. Rocky Mountain spotted fever — changing ecology and persisting virulence. N. Engl. J. Med. 353, 551–553 (2005).
Dantas-Torres, F. Rocky Mountain spotted fever. Lancet Infect. Dis. 7, 724–732 (2007).
Walker, D. H., Occhino, C., Tringali, G. R., Di Rosa, S. & Mansueto, S. Pathogenesis of rickettsial eschars: the tache noire of boutonneuse fever. Hum. Pathol. 19, 1449–1454 (1988).
Kaplowitz, L. G., Fischer, J. J. & Sparling, P. F. Rocky Mountain spotted fever: a clinical dilemma. Curr. Clin. Top. Infect. Dis. 2, 89–108 (1981).
Walker, D. H. & Raoult, D. in Principles and Practice of Infectious Diseases 6th edn (eds Mandell, G. L., Bennett, J. E. & Dolin, R.) 2287–2295 (Churchill Livingstone, New York, 2004).
Zinsser, H. Rats, Lice and History 3–301 (Little, Brown and Company, Boston, 1935).
Brill, N. E. An acute infectious disease of unknown origin: a clinical study based on 221 cases. Am. J. Med. Sci. 139, 484–502 (1910).
Zinsser, H. & Castaneda, M. R. On the isolation from a case of Brill's disease of a typhus strain resembling the European type. N. Engl. J. Med. 209, 815–819 (1933).
Biological and chemical terrorism: strategic plan for preparedness and response; recommendations of the CDC Strategic Planning Workgroup. MMWR 49, 1–14 (2000).
Alibek, K. & Handelman, S. Biohazard 3–319 (Random House, New York, 1999).
Reynolds, M. G. et al. Flying squirrel-associated typhus, United States. Emerg. Infect. Dis. 9, 1341–1343 (2003).
Medina-Sanchez, A. et al. Detection of a typhus group Rickettsia in Amblyomma ticks in the state of Nuevo Leon, Mexico. Ann. NY Acad. Sci. 1063, 327–332 (2005).
Reiss-Gutfreund, R. J. The isolation of Rickettsia prowazekii and mooseri from unusual sources. Am. J. Trop. Med. Hyg. 15, 943–950 (1966).
Burgdorfer, W., Hayes, S. F. & Mavros, A. J. in Rickettsiae and Rickettsial Diseases (eds Burgdorfer, W. & Anacker, R. L.) 585–594 (Academic, New York,1981).
Macaluso, K. R., Sonenshine, D. E., Ceraul, S. M. & Azad, A. F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39, 809–813 (2002).
Parker, R. R. & Spencer, R. R. Rocky Mountain spotted fever: a study of the relationship between the presence of rickettsia-like organisms in tick smears and infectiveness of the same ticks. Public Health Rep. 41, 461–469 (1926).
Burgdorfer, W. in Biology of Rickettsial Diseases (ed. Walker, D. H.) 33–50 (CRC, Boca Raton, 1988).
Niebylski, M. L., Peacock, M. G. & Schwan, T. G. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65, 773–778 (1999). First experimental proof that virulent Rickettsia spp. are pathogenic for their tick hosts, which revealed that rickettsial evolution of virulence has costs for both vertebrate and invertebrate hosts.
Walker, D. H., Harrison, A., Henderson, F. & Murphy, F. A. Identification of Rickettsia rickettsii in a guinea pig model by immunofluorescent and electron microscopic techniques. Am. J. Pathol. 86, 343–358 (1977).
Hayes, S. F. & Burgdorfer, W. Reactivation of Rickettsia rickettsii in Dermacentor andersoni ticks: an ultrastructural analysis. Infect. Immun. 37, 779–785 (1982).
Kotsyfakis, M. et al. Anti-inflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem. 281, 26298–26307 (2006).
Brossard, M. & Wikel, S. K. Tick immunobiology. Parasitology 129, S161–S176 (2004).
Valenzuela, J. G. Exploring tick saliva: from biochemistry to “sialomes'' and functional genomics. Parasitology 129, S83–S94 (2004).
Montgomery, R. R., Lusitani, D., De Boisfleury Chevance, A. & Malawista, S. E. Tick saliva reduces adherence and area of human neutrophils. Infect. Immun. 72, 2989–2994 (2004).
Kovar, L. Tick saliva in anti-tick immunity and pathogen transmission. Folia Microbiol. 49, 327–332 (2004).
Kubes, M., Fuchsberger, N., Labuda, M., Zuffova, E. & Nuttall, P. A. Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology 82, 113–116 (1994).
Gillespie, R. D., Dolan, M. C., Piesman, J. & Titus, R. G. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J. Immunol. 166, 4319–4326 (2001).
Ferreira, B. R. & Silva, J. S. Successive tick infestations selectively promote a T-helper 2 cytokine profile in mice. Immunology 96, 434–439 (1999).
Mejri, N. & Brossard, M. Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naive CD4+T to induce Th2 cell differentiation in vitro and in vivo. Int. Immunol. 19, 535–543 (2007).
Cavassani, K. A., Aliberti, J. C., Dias, A. R., Silva, J. S. & Ferreira, B. R. Tick saliva inhibits differentiation, maturation and function of murine bone-marrow-derived dendritic cells. Immunology 114, 235–245 (2005).
Sa-Nunes, A. et al. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol. 179, 1497–1505 (2007).
Martinez, J. J., Seveau, S., Veiga, E., Matsuyama, S. & Cossart, P. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell 123, 1013–1023 (2005). The first paper to identify the Ku70 subunit of DNA-dependent protein kinase as a cell-surface receptor that is used by spotted-fever-group rickettsiae to enter non-phagocytic cells. Also identified the rickettsial protein OmpB as a ligand for Ku70.
Ramm, L. E. & Winkler, H. H. Identification of cholesterol in the receptor site for rickettsiae on sheep erythrocyte membranes. Infect. Immun. 13, 120–126 (1976).
Martinez, J. J. & Cossart, P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J. Cell Sci. 117, 5097–5106 (2004).
Monferran, S. et al. The membrane-associated form of the DNA repair protein Ku is involved in cell adhesion to fibronectin. J. Mol. Biol. 337, 503–511 (2004).
Hackstadt, T., Messer, R., Cieplak, W. & Peacock, M. G. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect. Immun. 60, 159–165 (1992).
Crocquet-Valdes, P. A. et al. Immunization with a portion of rickettsial outer membrane protein A stimulates protective immunity against spotted fever rickettsiosis. Vaccine 20, 979–988 (2001).
Feng, H. M., Whitworth, T., Olano, J. P., Popov, V. L. & Walker, D. H. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 72, 2222–2228 (2004). Surprisingly, the long-standing emphasis on the importance of cell-mediated immunity for the clearance of rickettsiae and recovery from infection had missed the important concept for vaccine development that the presence of antibodies to epitopes of the outer-membrane proteins A and B prevents illness.
Uchiyama, T., Kawano, H. & Kusuhara, Y. The major outer membrane protein rOmpB of spotted fever group rickettsiae functions in the rickettsial adherence to and invasion of Vero cells. Microbes Infect. 8, 801–809 (2006).
Li, H. & Walker, D. H. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24, 289–298 (1998).
Jensenius, M. et al. Systemic inflammatory responses in African tick-bite fever. J. Infect. Dis. 187, 1332–1336 (2003).
Elghetany, M. T. & Walker, D. H. Hemostatic changes in Rocky Mountain spotted fever and Mediterranean spotted fever. Am. J. Clin. Pathol. 112, 159–168 (1999).
Teysseire, N., Arnoux, D. l., George, F., Sampol, J. & Raoult, D. von Willebrand factor release and thrombomodulin and tissue factor expression in Rickettsia conorii-infected endothelial cells. Infect. Immun. 60, 4388–4393 (1992).
Sporn, L. A. & Marder, V. J. Interleukin-1α production during Rickettsia rickettsii infection of cultured endothelial cells: potential role in autocrine cell stimulation. Infect. Immun. 64, 1609–1613 (1996).
Shi, R.-J., Simpson-Haidaris, P. J., Marder, V. J., Silverman, D. J. & Sporn, L. A. Increased expression of plasminogen activator inhibitor-1 in R. rickettsii-infected endothelial cells. Thromb. Haemost. 75, 600–606 (1996).
Oristrell, J., Sampere, M., Amengual, M. J., Font, B. & Segura, F. Plasma interleukin-6 levels in Mediterranean spotted fever. Eur. J. Clin. Microbiol. Infect. Dis. 23, 417–418 (2004).
Rydkina, E., Sahni, A., Baggs, R. B., Silverman, D. J. & Sahni, S. K. Infection of human endothelial cells with spotted fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins. Infect. Immun. 74, 5067–5074 (2006).
Walker, T. S., Brown, J. S., Hoover, C. S. & Morgan, D. A. Endothelial prostaglandin secretion: effects of typhus rickettsiae. J. Infect. Dis. 162, 1136–1144 (1990).
Rydkina, E., Sahni, A., Silverman, D. J. & Sahni, S. K. Rickettsia rickettsii infection of cultured human endothelial cells induces heme oxygenase 1 expression. Infect. Immun. 70, 4045–4052 (2002).
Woods, M. E. & Olano, J. P. Host defenses to Rickettsia rickettsii infection contribute to increased microvascular permeability in human cerebral endothelial cells. J. Clin. Immunol. 28, 174–185 (2008). The most important pathophysiological effect of rickettsial disease, increased vascular permeability, was addressed mechanistically, which revealed the likelihood of cytokine contribution to pathology.
Clifton, D. R. et al. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc. Natl Acad. Sci. USA 95, 4646–4651 (1998).
Clifton, D. R., Rydkina, E., Freeman, R. S. & Sahni, S. K. NF-κB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IκB kinases IKKα and IKKβ and phosphorylation-proteolysis of the inhibitor protein IκBα. Infect. Immun. 73, 155–165 (2005).
Valbuena, G., Bradford, W. & Walker, D. H. Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group. Am. J. Pathol. 163, 1357–1369 (2003).
Valbuena, G. & Walker, D. H. Expression of CX3CL1 (fractalkine) in mice with endothelial-target rickettsial infection of the spotted-fever group. Virchows Archiv. 446, 21–27 (2004).
Whitworth, T., Popov, V. L., Yu, X. J., Walker, D. H. & Bouyer, D. H. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect. Immun. 73, 6668–6673 (2005).
Monack, D. M. & Theriot, J. A. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell. Microbiol. 3, 633–647 (2001).
Heinzen, R. A., Grieshaber, S. S., Van Kirk, L. S. & Devin, C. J. Dynamics of actin-based movement by Rickettsia rickettsii in Vero cells. Infect. Immun. 67, 4201–4207 (1999).
Van Kirk, L. S., Hayes, S. F. & Heinzen, R. A. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect. Immun. 68, 4706–4713 (2000).
Gouin, E. et al. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427, 457–461 (2004). Rickettsial actin-based mobility was shown to be associated with the activation of Arp2/3 by Rick A, a protein of the spotted-fever-group rickettsiae.
Jeng, R. L. et al. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell. Microbiol. 6, 761–769 (2004).
Simser, J. A., Rahman, M. S., Dreher-Lesnick, S. M. & Azad, A. F. A novel and naturally occurring transposon, ISRpe1 in the Rickettsia peacockii genome disrupting the rickA gene involved in actin-based motility. Mol. Microbiol. 58, 71–79 (2005).
Feng, H. M., Popov, V. L. & Walker, D. H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 62, 1952–1960 (1994).
Walker, D. H., Olano, J. P. & Feng, H. M. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect. Immun. 69, 1841–1846 (2001). Revealed that recovery from rickettsial infection requires the CD8+ T lymphocyte cytotoxic response.
Sousa, R. et al. Intralesional expression of mRNA of interferon-γ, tumour necrosis factor-α, interleukin-10, nitric oxide synthase, indoleamine-2,3-dioxygenase, and RANTES is a major immune effector in Mediterranean spotted fever rickettsiosis. J. Infect. Dis. 196, 770–781 (2007). The important immune effectors in rickettsial infections of mice were demonstrated to be present in lesions of human patients with Mediterranean spotted fever.
Feng, H. M. & Walker, D. H. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 68, 6729–6736 (2000). Rickettsicidal mechanisms that are important in intracellular killing in mice — nitric oxide, reactive oxygen species and tryptophan limitation — were demonstrated to be effectors in human target cells.
Fang, R. et al. Differential interaction of dendritic cells with Rickettsia: impact on host susceptibility to murine spotted fever rickettsiosis. Infect. Immun. 75, 3112–3123 (2007).
Jordan, J. M., Woods, M. E., Feng, H.-M., Soong, L. & Walker, D. H. Rickettsiae-stimulated dendritic cells mediate protection against lethal rickettsial challenge in an animal model of spotted fever rickettsiosis. J. Infect. Dis. 196, 629–638 (2007). Revealed that DCs play crucial parts in innate and adaptive immunity to Rickettsia spp.
Billings, A. N., Feng, H. M., Olano, J. P. & Walker, D. H. Rickettsial infection in murine models activates an early anti-rickettsial effect mediated by NK cells and associated with production of gamma interferon. Am. J. Trop. Med. Hyg. 65, 52–56 (2001).
Feng, H. M., Popov, V. L., Yuoh, G. & Walker, D. H. Role of T lymphocyte subsets in immunity to spotted fever group rickettsiae. J. Immunol. 158, 5314–5320 (1997).
Herrero-Herrero, J. I., Walker, D. H. & Ruiz-Beltran, R. Immunohistochemical evaluation of the cellular immune response to Rickettsia conorii in taches noires. J. Infect. Dis. 155, 802–805 (1987).
Feng, H. M., Whitworth, T., Popov, V. L. & Walker, D. H. Effect of antibody on the rickettsia–host cell interaction. Infect. Immun. 72, 3524–3530 (2004).
Carl, M. et al. Heterogeneity of CD4-positive human T-cell clones which recognize the surface protein antigen of Rickettsia typhi. Infect. Immun. 57, 1276–1280 (1989).
Ching, W. M., Carl, M. & Dasch, G. A. Mapping of monoclonal antibody binding sites on CNBr fragments of the S-layer protein antigens of Rickettsia typhi and Rickettsia prowazekii. Mol. Immunol. 29, 95–105 (1992).
Ching, W. M., Wang, H., Jan, B. & Dasch, G. A. Identification and characterization of epitopes on the 120-kilodalton surface protein antigen of Rickettsia prowazekii with synthetic peptides. Infect. Immun. 64, 1413–1419 (1996).
Ellison, D. W. et al. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infect. Immun. 76, 542–550 (2008).
Sumner, J. W., Sims, K. G., Jones, D. C. & Anderson, B. E. Protection of guinea-pigs from experimental Rocky Mountain spotted fever by immunization with baculovirus-expressed Rickettsia rickettsii rOmpA protein. Vaccine 13, 29–35 (1995). Showed that outer-membrane protein A is an antigen that stimulates protective immunity to R. rickettsii , and thus is an excellent candidate for incorporation into a protective vaccine against SFG rickettsiae.
Bourgeois, A. L. & Dasch, G. A. in Rickettsiae and Rickettsial Diseases (eds Burgdorfer, W. & Anacker, R. L.) 71–80 (Academic, New York, 1981). Showed that outer-membrane protein B is an antigen that stimulates protective immunity to R. typhi , and thus is an excellent candidate for incorporation into vaccines against all Rickettsia spp.
Valbuena, G., Jordan, J. M., Walker, D. H. T cells mediate cross-protective immunity between spotted fever group rickettsiae and typhus group rickettsiae. J. Infect. Dis. 190, 1221–1227 (2004). Divergent Rickettsia spp. of the typhus and spotted-fever groups that generally do not stimulate even cross-reactive antibodies were shown to contain antigens that stimulate T lymphocytes, which mediate cross-protection.
Vitorino, L., Chelo, I. M., Bacellar, F. & Ze-Ze, L. Rickettsial phylogeny: a multigenic approach. Microbiology 153, 160–168 (2007).
Gevers, D. et al. Re-evaluating prokaryotic species. Nature Rev. Microbiol. 3, 733–739 (2005).
Andersson, J. O. & Andersson, S. G. Insights into the evolutionary process of genome degradation. Curr. Opin. Genet. Dev. 9, 664–671 (1999).
Andersson, S. G. & Kurland, C. G. Origins of mitochondria and hydrogenosomes. Curr. Opin. Microbiol. 2, 535–541 (1999).
Andersson, S. G. et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396, 133–140 (1998).
Ogata, H. et al. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293, 2093–2098 (2001).
Ogata, H., Renesto, P., Audic, S., Robert, C. & Blanc, G. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 3, e248 (2005).
Baldridge, G. D., Burkhardt, N. Y., Felsheim, R. F., Kurtti, T. J. & Munderloh, U. G. Transposon insertion reveals pRM, a plasmid of Rickettsia monacensis. Appl. Environ. Microbiol. 73, 4984–4995 (2007).
Baldridge, G. D., Burkhardt, N. Y., Felsheim, R. F., Kurtti, T. J. & Munderloh, U. G. Plasmids of the pRM/pRF family occur in diverse Rickettsia species. Appl. Environ. Microbiol. 74, 645–652 (2008).
Andersson, J. O. & Andersson, S. G. Pseudogenes, junk DNA, and the dynamics of Rickettsia genomes. Mol. Biol. Evol. 18, 829–839 (2001).
Kana, B. D. et al. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 183, 7076–7086 (2001).
Renesto, P. et al. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J. Infect. Dis. 188, 1276–1283 (2003). A potentially important virulence factor of pathogenic rickettsiae, phospholipase D, was identified and characterized functionally.
Gaywee, J., Xu, W., Radulovic, S., Bessman, M. J. & Azad, A. F. The Rickettsia prowazekii invasion gene homolog (invA) encodes a nudix hydrolase active on adenosine (5′)-pentaphospho-(5′)-adenosine. Mol. Cell. Proteomics 1, 179–185 (2002).
Gaywee, J., Radulovic, S., Higgins, J. A. & Azad, A. F. Transcriptional analysis of Rickettsia prowazekii invasion gene homolog (invA) during host cell infection. Infect. Immun. 70, 6346–6354 (2002).
Ngwamidiba, M., Blanc, G., Raoult, D. & Fournier, P. E. Sca1, a previously undescribed paralog from autotransporter protein-encoding genes in Rickettsia species. BMC Microbiol. 6, 12 (2006).
Ogawa, M. et al. Proteome analysis of Rickettsia felis highlights the expression profile of intracellular bacteria. Proteomics 7, 1232–1248 (2007).
Burns, D. L. Type IV transporters of pathogenic bacteria. Curr. Opin. Microbiol. 6, 29–34 (2003).
Acknowledgements
The authors thank D. Baker for expert secretarial contributions.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez Genome Project
Rickettsia rickettsiistr. Iowa
Rickettsia rickettsiistr.Sheila Smith
FURTHER INFORMATION
CDC emergency preparedness and response: bioterrorism agents and diseases (by category)
Glossary
- Housekeeping gene
-
A gene that is involved in the basic functions that are required for normal cell metabolism and is constitutively expressed.
- Hypovolaemia
-
Decreased blood volume more specifically, a decrease in the volume of blood plasma.
- Hypotensive shock
-
Shock in which blood pressure is lower than normal and does not supply blood to the organs.
- Superinfection
-
Infection by a microorganism of a cell that is already infected by another microorganism.
- Transovarial
-
Passage of parasites or infective agents from the maternal body to eggs within the ovaries and subsequently to the larvae that hatch from the eggs.
- Trans-stadial
-
Passage of a microorganism from one developmental stage (stadium) of the host to a subsequent stage (or stages).
- Haemostatic
-
Stops blood flow.
- Vasoactive
-
Causes constriction or dilation of blood vessels.
Rights and permissions
About this article
Cite this article
Walker, D., Ismail, N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol 6, 375–386 (2008). https://doi.org/10.1038/nrmicro1866
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1866
This article is cited by
-
Meningoencephalitis and retinal vasculitis due to rickettsial infection
Journal of Neurology (2023)
-
Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle
Nature Reviews Microbiology (2021)
-
Microbial communities associated with the camel tick, Hyalomma dromedarii: 16S rRNA gene-based analysis
Scientific Reports (2020)
-
Deianiraea, an extracellular bacterium associated with the ciliate Paramecium, suggests an alternative scenario for the evolution of Rickettsiales
The ISME Journal (2019)
-
Evasion of autophagy mediated by Rickettsia surface protein OmpB is critical for virulence
Nature Microbiology (2019)