Key Points
-
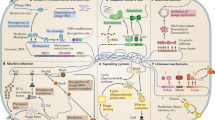
Phages (bacterial viruses) are the most numerous microorganisms on Earth. Bacteria have developed an astonishing array of strategies to combat these viruses at each step of the infection process. Here we describe these strategies and how phages have adapted to subvert them.
-
Phage adsorption to cell receptors is the initial step of infection, and some bacterial strains have developed mechanisms to prevent this key process. There are at least three strategies used by adsorption-blocking systems: receptor blocking, extracellular matrix production and competitive inhibitor production.
-
Superinfection exclusion (Sie) systems prevent phage DNA entry into the host cell, thereby conferring bacterial immunity against superinfecting phages. Several mechanisms that inhibit phage DNA injection have been uncovered in Gram-negative and Gram-positive bacteria.
-
The notorious bacterial restriction–modification systems prevent phage infection by cleaving phage genomic DNA. As a response, phages have evolved by specifically modifying their genomes to avoid DNA cleavage. This ongoing battle of co-evolution between bacteria and phages is exemplified by the canonical coliphage T4.
-
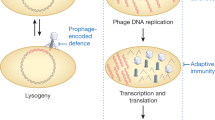
Clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR-associated (cas) genes are new fascinating topics. The bacterial and archeal CRISPR–Cas systems confer immunity against incoming foreign DNA such as phage genomes. A likely mode of action of this mechanism has been proposed, along with phage strategies to circumvent this system.
-
Bacteria have also evolved a plethora of intracellular proteins that cause abortion of the phage infection. These antiphage mechanisms target crucial steps of phage multiplication, such as transcription, protein synthesis, maturation and host cell lysis. Several different abortive infection (Abi) systems have been found, and the mode of action has been studied for a few of these.
Abstract
Phages are now acknowledged as the most abundant microorganisms on the planet and are also possibly the most diversified. This diversity is mostly driven by their dynamic adaptation when facing selective pressure such as phage resistance mechanisms, which are widespread in bacterial hosts. When infecting bacterial cells, phages face a range of antiviral mechanisms, and they have evolved multiple tactics to avoid, circumvent or subvert these mechanisms in order to thrive in most environments. In this Review, we highlight the most important antiviral mechanisms of bacteria as well as the counter-attacks used by phages to evade these systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brüssow, H. & Hendrix, R. W. Phage genomics: small is beautiful. Cell 108, 13–16 (2002).
Okafor, N. Modern Industrial Microbiology and Biotechnology (Science Publishers, Enfield, New Hampshire, 2007).
Hutkins, R. W. Microbiology and Technology of Fermented Foods (Blackwell Publishing, Chicago, 2006).
Émond, É. & Moineau, S. in Bacteriohpage: Genetics and Molecular Biology (eds McGrath, S. & Van Sinderen, D.) 93–123 (Caister Academic, Norwich, Norfolk, 2007).
Sturino, J. M. & Klaenhammer, T. R. Engineered bacteriophage-defence systems in bioprocessing. Nature Rev. Microbiol. 4, 395–404 (2006).
O'Flaherty, S., Ross, R. P. & Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 33, 801–819 (2009).
Mattey, M. & Spencer, J. Bacteriophage therapy — cooked goose or Phoenix rising? Curr. Opin. Biotechnol. 19, 608–612 (2008).
Hanlon, G. W. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrobi. Agents 30, 118–128 (2007).
Campbell, A. The future of bacteriophage biology. Nature Rev. Genet. 4, 471–477 (2003).
Sulakvelidze, A., Alavidze, Z. & Morris, J. G. Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659 (2001).
Foster, T. J. Immune evasion by staphylococci. Nature Rev. Microbiol. 3, 948–958 (2005).
Nordström, K. & Forsgren, A. Effect of protein A on adsorption of bacteriophages to Staphylococcus aureus. J. Virol. 14, 198–202 (1974).
Pedruzzi, I., Rosenbusch, J. P. & Locher, K. P. Inactivation in vitro of the Escherichia coli outer membrane protein FhuA by a phage T5-encoded lipoprotein. FEMS Microbiol. Lett. 168, 119–125 (1998).
Riede, I. & Eschbach, M. L. Evidence that TraT interacts with OmpA of Escherichia coli. FEBS Lett. 205, 241–245 (1986).
Uhl, M. A. & Miller, J. F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BVgAS phosphorelay. EMBO J. 15, 1028–1036 (1996).
Beier, D. & Gross, R. in Bacterial Signal Transduction: Networks and Drug Targets. (ed. Utsumi, R.) 149–160 (Springer, New York, 2008).
Liu, M. et al. Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295, 2091–2094 (2002). This fascinating paper describes how phages infecting Bordetella spp. can adapt to the cell surface variations that occur in their hosts in different environmental conditions.
Doulatov, S. et al. Tropism switching in Bordetella bacteriophage defines a family of diversity-generating retroelements. Nature 431, 476–481 (2004).
Medhekar, B. & Miller, J. F. Diversity-generating retroelements. Curr. Opin. Microbiol. 10, 388–395 (2007).
Ventura, M. et al. Prophage-like elements in bifidobacteria: insights from genomics, transcription, integration, distribution, and phylogenetic analysis. Appl. Environ. Microbiol. 71, 8692–8705 (2005).
Stummeyer, K. et al. Evolution of bacteriophages infecting encapsulated bacteria: lessons from Escherichia coli K1-specific phages. Mol. Microbiol. 60, 1123–1135 (2006).
Sutherland, I. W. Polysaccharide lyases. FEMS Microbiol. Rev. 16, 323–347 (1995). This is the most complete review on the topic of the enzymes that degrade polysaccharides.
Sutherland, I. W. Polysaccharases for microbial exopolysaccharides. Carbohydr. Polym. 38, 319–328 (1999).
Sutherland, I. W., Hughes, K. A., Skillman, L. C. & Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 232, 1–6 (2004).
Linhardt, R. J., Galliher, P. M. & Cooney, C. L. Polyccharide lyases. Appl. Biochem. Biotechnol. 12, 135–176 (1986).
Hammad, A. M. M. Evaluation of alginate-encapsulated Azotobacter chroococcum as a phage-resistant and an effective inoculum. J. Basic Microbiol. 38, 9–16 (1998).
Hanlon, G. W., Denyer, S. P., Olliff, C. J. & Ibrahim, L. J. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 67, 2746–2753 (2001).
Castillo, F. J. & Bartell, P. F. Studies on bacteriophage-2 receptors of Pseudomonas aeruginosa. J. Virol. 14, 904–909 (1974).
Castillo, F. J. & Bartell, P. F. Localization and functional role of Pseudomonas bacteriophage 2 depolymerase. J. Virol. 18, 701–708 (1976).
Temple, G. S., Ayling, P. D. & Wilkinson, S. G. Isolation and characterization of a lipopolysaccharide-specific bacteriophage of Pseudomonas aeruginosa. Microbios 45, 81–91 (1986).
Hynes, W. L., Hancock, L. & Ferretti, J. J. Analysis of a 2nd bacteriophage hyaluronidase gene from Streptococcus pyogenes: evidence for a 3rd hyaluronidase involved in extracellular enzymatic activity. Infect. Immun. 63, 3015–3020 (1995).
Boulnois, G. J. & Roberts, I. S. Genetics of capsular polysaccharide production in bacteria. Curr. Top. Microbiol. Immunol. 150, 1–18 (1990).
Moses, A. E. et al. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect. Immun. 65, 64–71 (1997).
McClean, D. The capsulation of streptococci and its relation to diffusion factor (hyaluronidase). J. Pathol. Bacteriol. 53, 13–27 (1941).
Kjems, E. Studies on streptococcal bacteriophages. I. Technique of isolating phage-producing strains. Acta Pathol. Microbiol. Scand. 36, 433–440 (1955).
Benchetrit, L. C., Gray, E. D. & Wannamaker, L. W. Hyaluronidase activity of bacteriophages of group A streptococci. Infect. Immun. 15, 527–532 (1977).
Stirm, S. Escherichia coli K bacteriophages, I. Isolation and introductory characterization of five Escherichia coli K bacteriophages. J. Virol. 2, 1107–1114 (1968).
Steinbacher, S., Miller, S., Baxa, U., Weintraub, A. & Seckler, R. Interaction of Salmonella phage P22 with its O-antigen receptor studied by X-ray crystallography. J. Biol. Chem. 378, 337–343 (1997).
Perry, L. L. et al. Sequence analysis of Escherichia coli O157:H7 bacteriophage ΦV10 and identification of a phage-encoded immunity protein that modifies the O157 antigen. FEMS Microbiol. Lett. 292, 182–186 (2009).
Zaleski, P., Wojciechowski, M. & Piekarowicz, A. The role of Dam methylation in phase variation of Haemophilus influenzae genes involved in defence against phage infection. Microbiology 151, 3361–3369 (2005).
Destoumieux-Garzon, D. et al. The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: role of the microcin Val11-Pro16 β-hairpin region in the recognition mechanism. Biochem. J. 389, 869–876 (2005).
Lu, M. J., Stierhof, Y. D. & Henning, U. Location and unusual membrane topology of the immunity protein of the Escherichia coli phage T4. J. Virol. 67, 4905–4913 (1993).
Lu, M. J. & Henning, U. Superinfection exclusion by T-even-type coliphages. Trends Microbiol. 2, 137–139 (1994). This paper describes the superinfection exclusion mechanisms that are found in phage T4.
Moak, M. & Molineux, I. J. Role of the Gp16 lytic transglycosylase motif in bacteriophage T7 virions at the initiation of infection. Mol. Microbiol. 37, 345–355 (2000).
Kliem, M. & Dreiseikelmann, B. The superimmunity gene sim of bacteriophage P1 causes superinfection exclusion. Virology 171, 350–355 (1989).
Maillou, J. & Dreiseikelmann, B. The sim gene of Escherichia coli phage P1: nucleotide sequence and purification of the processed protein. Virology 175, 500–507 (1990).
Hofer, B., Ruge, M. & Dreiseikelmann, B. The superinfection exclusion gene (sieA) of bacteriophage P22: identification and overexpression of the gene and localization of the gene product. J. Bacteriol. 177, 3080–3086 (1995).
Garvey, P., Hill, C. & Fitzgerald, G. The lactococcal plasmid pNP40 encodes a third bacteriophage resistance mechanism, one which affects phage DNA penetration. Appl. Environ. Microbiol. 62, 676–679 (1996).
Akçelik, M. A phage DNA injection-blocking type resistance mechanism encoded by chromosomal DNA in Lactococcus lactis subsp. lactis PLM-18. Milchwissenschaft 53, 619–622 (1998).
McGrath, S., Fitzgerald, G. F. & van Sinderen, D. Identification and characterization of phage-resistance genes in temperate lactococcal bacteriophages. Mol. Microbiol. 43, 509–520 (2002).
Mahony, J., McGrath, S., Fitzgerald, G. F. & van Sinderen, D. Identification and characterization of lactococcal-prophage-carried superinfection exclusion genes. Appl. Environ. Microbiol. 74, 6206–6215 (2008).
Sun, X., Gohler, A., Heller, K. J. & Neve, H. The ltp gene of temperate Streptococcus thermophilus phage TP-J34 confers superinfection exclusion to Streptococcus thermophilus and Lactococcus lactis. Virology 350, 146–157 (2006).
Pingoud, A., Fuxreiter, M., Pingoud, V. & Wende, W. Type II restriction endonucleases: structure and mechanism. Cell. Mol. Life Sci. 62, 685–707 (2005).
Pingoud, A. M. Restriction Endonucleases (Springer, Berlin, 2004).
Kruger, D. H. & Bickle, T. A. Bacteriophage survival: multiple mechanisms for avoiding the deoxyribonucleic-acid restriction systems of their hosts. Microbiol. Rev. 47, 345–360 (1983).
Kruger, D. H., Barcak, G. J. & Smith, H. O. Abolition of DNA recognition site resistance to the restriction endonuclease EcoRII. Biomed. Biochim. Acta 47, K1–K5 (1988).
Tock, M. R. & Dryden, D. T. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Wilson, G. G. & Murray, N. E. Restriction and modification systems. Annu. Rev. Genet. 25, 585–627 (1991).
McGrath, S., Seegers, J. F. M. L., Fitzgerald, G. F. & van Sinderen, D. Molecular characterization of a phage-encoded resistance system in Lactococcus lactis. Appl. Environ. Microbiol. 65, 1891–1899 (1999).
Bickle, T. A. & Kruger, D. H. Biology of DNA restriction. Microbiol. Rev. 57, 434–450 (1993).
Vovis, G. F. & Lacks, S. Complementary action of restriction enzymes endo R-DpnI and endo R-DpnII on bacteriophage-f1 DNA. J. Mol. Biol. 115, 525–538 (1977).
Raleigh, E. A. & Wilson, G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc. Natl Acad. Sci. USA 83, 9070–9074 (1986).
Bair, C. L. & Black, L. W. A type IV modification dependent restriction nuclease that targets glucosylated hydroxymethyl cytosine modified DNAs. J. Mol. Biol. 366, 768–778 (2007). This article describes how phage T4 subverts the GmrS–GmrD system (which specifically cleaves glucosyl-hydroxymethylcytosine-modified DNA) using an injected protein inhibitor.
Bair, C. L., Rifat, D. & Black, L. W. Exclusion of glucosyl-hydroxymethylcytosine DNA containing bacteriophages is overcome by the injected protein inhibitor IPI*. J. Mol. Biol. 366, 779–789 (2007).
Rifat, D., Wright, N. T., Varney, K. M., Weber, D. J. & Black, L. W. Restriction endonuclease inhibitor IPI* of bacteriophage T4: a novel structure for a dedicated target. J. Mol. Biol. 375, 720–734 (2008).
Zabeau, M., Friedman, S., Montagu, M. V. & Schell, J. The ral gene of phage λ.1. Identification of a non-essential gene that modulates restriction and modification in Escherichia coli. Mol. Gen. Genet. 179, 63–73 (1980).
Zissler, J., Singer, E. R. & Schaefer, F. in The Bacteriophage λ (ed. Hershey, A. D.) 455–475 (Cold Spring Harbor Laboratory Press, New York, 1971).
King, G. & Murray, N. E. Restriction alleviation and modification enhancement by the Rac prophage of Escherichia coli K-12. Mol. Microbiol. 16, 769–777 (1995).
Toothman, P. Restriction alleviation by bacteriophages lambda and lambda reverse. J. Virol. 38, 621–631 (1981).
Walkinshaw, M. D. et al. Structure of Ocr from bacteriophage T7, a protein that mimics B-form DNA. Mol. Cell 9, 187–194 (2002).
Studier, F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J. Mol. Biol. 79, 237–248 (1973).
Atanasiu, C., Su, T. J., Sturrock, S. S. & Dryden, D. T. Interaction of the ocr gene 0.3 protein of bacteriophage T7 with EcoKI restriction/modification enzyme. Nucleic Acids Res. 30, 3936–3944 (2002).
Bandyopadhyay, P. K., Studier, F. W., Hamilton, D. L. & Yuan, R. Inhibition of the type I restriction-modification enzymes EcoB and EcoK by the gene 0.3 protein of bacteriophage T7. J. Mol. Biol. 182, 567–578 (1985).
Kennaway, C. K. et al. The structure of M.EcoKI type I DNA methyltransferase with a DNA mimic antirestriction protein. Nucleic Acids Res. 37, 762–770 (2009).
Studier, F. W. & Movva, N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J. Virol. 19, 136–145 (1976).
Sistla, S. & Rao, D. N. S-Adenosyl-L-methionine-dependent restriction enzymes. Crit. Rev. Biochem. Mol. Biol. 39, 1–19 (2004).
Iida, S., Streiff, M. B., Bickle, T. A. & Arber, W. Two DNA antirestriction systems of bacteriophage P1, darA, and darB: characterization of darA− phage. Virology 157, 156–166 (1987).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007). The first evidence that the CRISPR–Cas system is an immunity system against foreign DNA.
Sorek, R., Kunin, V. & Hugenholtz, P. CRISPR — a widespread system that provides acquired resistance against phages in bacteria and archaea. Nature Rev. Microbiol. 6, 181–186 (2008).
Marraffini, L. A. & Sontheimer, E. J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463, 568–571 (2010).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nature Rev. Genet. 11, 181–190 (2010).
Karginov, F. V. & Hannon, G. J. The CRISPR system: small RNA-guided defense in bacteria and archaea. Mol. Cell 37, 7–19 (2010).
Horvath, P. & Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 327, 167–170 (2010).
Ishino, Y., Shinagawa, H., Makino, K., Amemura, M. & Nakata, A. Nucleotide-sequence of the iap gene, responsible for alkaline-phosphatase isozyme conversion in Escherichia coli, and identification of the gene-product. J. Bacteriol. 169, 5429–5433 (1987).
Godde, J. S. & Bickerton, A. The repetitive DNA elements called CRISPRs and their associated genes: evidence of horizontal transfer among prokaryotes. J. Mol. Evol. 62, 718–729 (2006).
Horvath, P. et al. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 190, 1401–1412 (2008).
Jansen, R., van Embden, J. D. A., Gaastra, W. & Schouls, L. M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 43, 1565–1575 (2002).
Makarova, K. S., Grishin, N. V., Shabalina, S. A., Wolf, Y. I. & Koonin, E. V. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct 1, 7 (2006).
Vestergaard, G. et al. Stygiolobus rod-shaped virus and the interplay of crenarchaeal rudiviruses with the CRISPR antiviral system. J. Bacteriol. 190, 6837–6845 (2008).
Bolotin, A., Ouinquis, B., Sorokin, A. & Ehrlich, S. D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151, 2551–2561 (2005).
Haft, D. H., Selengut, J., Mongodin, E. F. & Nelson, K. E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 1, 474–483 (2005).
Mojica, F. J. M., Diez-Villasenor, C., Garcia-Martinez, J. & Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182 (2005).
Pourcel, C., Salvignol, G. & Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151, 653–663 (2005).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008). This study shows that the CRISPR–Cas system targets foreign DNA.
Zegans, M. E. et al. Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of P. aeruginosa. J. Bacteriol. 191, 210–219 (2009).
Mojica, F. J. M., Diez-Villasenor, C., Garcia-Martinez, J. & Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155, 733–740 (2009).
Brouns, S. J. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008). A demonstration that one of the cas genes encodes an RNA endonuclease that is required for the CRISPR–Cas system to be active against phages.
Tang, T. H. et al. Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidus. Proc. Natl Acad. Sci. USA 99, 7536–7541 (2002).
Tang, T. H. et al. Identification of novel non-coding RNAs as potential antisense regulators in the archaeon Sulfolobus solfataricus. Mol. Microbiol. 55, 469–481 (2005).
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400 (2008).
Andersson, A. F. & Banfield, J. F. Virus population dynamics and acquired virus resistance in natural microbial communities. Science 320, 1047–1050 (2008).
Molineux, I. J. Host-parasite interactions: recent developments in the genetics of abortive phage infections. New Biol. 3, 230–236 (1991). A comprehensive review of the phage exclusion systems in E. coli.
Snyder, L. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol. 15, 415–420 (1995). A summary of the progress that has been made in understanding the modes of action of phage exclusion systems in Gram-negative bacteria.
Parma, D. H. et al. The Rex system of bacteriophage lambda: tolerance and altruistic cell death. Genes Dev. 6, 497–510 (1992).
Snyder, L. & McWilliams, K. The rex genes of bacteriophage lambda can inhibit cell function without phage superinfection. Gene 81, 17–24 (1989).
Hinton, D. M. et al. Transcriptional takeover by σ appropriation: remodelling of the σ70 subunit of Escherichia coli RNA polymerase by the bacteriophage T4 activator MotA and co-activator AsiA. Microbiology 151, 1729–1740 (2005).
Kaufmann, G. Anticodon nucleases. Trends Biochem. Sci. 25, 70–74 (2000).
Bingham, R., Ekunwe, S. I. N., Falk, S., Snyder, L. & Kleanthous, C. The major head protein of bacteriophage T4 binds specifically to elongation factor Tu. J. Biol. Chem. 275, 23219–23226 (2000).
Cheng, X., Wang, W. & Molineux, I. J. F exclusion of bacteriophage T7 occurs at the cell membrane. Virology 326, 340–352 (2004).
Garcia, L. R. & Molineux, I. J. Incomplete entry of bacteriophage T7 DNA into F-plasmid containing Escherichia coli. J. Bacteriol. 177, 4077–4083 (1995).
Schmitt, C. K., Kemp, P. & Molineux, I. J. Genes 1.2 and 10 of bacteriophages T3 and T7 determine the permeability lesions observed in infected cells of Escherichia coli expressing the F plasmid gene pifA. J. Bacteriol. 173, 6507–6514 (1991).
Chopin, M.-C., Chopin, A. & Bidnenko, E. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8, 473–479 (2005). This article is the most complete review on Abi mechanism in L. lactis.
Hill, C., Miller, L. A. & Klaenhammer, T. R. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl. Environ. Microbiol. 56, 2255–2258 (1990).
Garvey, P., Fitzgerald, G. F. & Hill, C. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl. Environ. Microbiol. 61, 4321–4328 (1995).
Émond, É. et al. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl. Environ. Microbiol. 63, 1274–1283 (1997).
Domingues, S., Chopin, A., Ehrlich, S. D. & Chopin, M.-C. The lactococcal abortive phage infection system AbiP prevents both phage DNA replication and temporal transcription switch. J. Bacteriol. 186, 713–721 (2004).
Bouchard, J. D., Dion, É., Bissonnette, F. & Moineau, Characterization of the two-component abortive phage infection mechanism AbiT from Lactococcus lactis. J. Bacteriol. 184, 6325–6332 (2002).
Dai, G. et al. Molecular characterization of a new abortive infection system (AbiU) from Lactococcus lactis LL51–51 Appl. Environ. Microbiol. 67, 5225–5232 (2001).
Cluzel, P. J., Chopin, A., Ehrlich, S. D. & Chopin, M.-C. Phage abortive infection mechanism from Lactococcus lactis subsp. lactis, expression of which is mediated by an Iso-ISS1 element. Appl. Environ. Microbiol. 57, 3547–3551 (1991).
O'Connor, L., Tangney, M. & Fitzgerald, G. F. Expression, regulation, and mode of action of the AbiG abortive infection system of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 65, 330–335 (1999).
Durmaz, E., Higgins, D. L. & Klaenhammer, T. R. Molecular characterization of a second abortive phage resistance gene present in Lactococcus lactis subsp. lactis ME2. J. Bacteriol. 174, 7463–7469 (1992).
Émond, É. et al. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl. Environ. Microbiol. 64, 4748–4756 (1998).
Bidnenko, E., Ehrlich, S. D. & Chopin, M.-C. Lactococcus lactis phage operon coding for an endonuclease homologous to RuvC. Mol. Microbiol. 28, 823–834 (1998).
Durmaz, E. & Klaenhammer, T. R. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189, 1417–1425 (2007).
Domingues, S., Chopin, A., Ehrlich, S. D. & Chopin, M.-C. A phage protein confers resistance to the lactococcal abortive infection mechanism AbiP. J. Bacteriol. 186, 3278–3281 (2004).
Domingues, S. et al. The lactococcal abortive infection protein AbiP is membrane-anchored and binds nucleic acids. Virology 373, 14–24 (2008).
Boucher, I., Émond, É., Dion, É., Montpetit, D. & Moineau, S. Microbiological and molecular impacts of AbiK on the lytic cycle of Lactococcus lactis phages of the 936 and P335 species. Microbiology 146, 445–453 (2000).
Bouchard, J. D. & Moineau, S. Lactococcal phage genes involved in sensitivity to AbiK and their relation to single-strand annealing proteins. J. Bacteriol. 186, 3649–3652 (2004).
Fortier, L.-C., Bouchard, J. D. & Moineau, S. Expression and site-directed mutagenesis of the lactococcal abortive phage infection protein AbiK. J. Bacteriol. 187, 3721–3730 (2005).
Fineran, P. C. et al. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc. Natl Acad. Sci. USA 106, 894–899 (2009). The research described in this paper is the most valuable study on the use of TA systems as antiphage mechanisms.
Magnuson, R. D. Hypothetical functions of toxin-antitoxin systems. J. Bacteriol. 189, 6089–6092 (2007).
Blower, T. R. et al. Mutagenesis and functional characterisation of the RNA and protein components of the toxIN abortive infection and toxin-antitoxin locus of Erwinia. J. Bacteriol. 191, 6029–6039 (2009).
Pecota, D. C. & Wood, T. K. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J. Bacteriol. 178, 2044–2050 (1996).
Hazan, R. & Engelberg-Kulka, H. Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1. Mol. Genet. Genomics 272, 227–234 (2004).
Norrby, E. Nobel Prizes and the emerging virus concept. Arch. Virol. 153, 1109–1123 (2008).
Acknowledgements
We thank B.-A. Conway for editorial assistance. S.J.L. was a recipient of a graduate scholarship from the Natural Sciences and Engineering Research Council (NSERC) of Canada. J.E.S. is a recipient of a graduate scholarship from the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT). S.M. would like to acknowledge the funding support of NSERC, FQRNT, the Canadian Institutes of Health Research, Novalait, Agropur and Danisco.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Phase variation
-
A genetically programmed biological phenomenon that occurs in bacteria that need to adapt to different environments. These bacteria can modify their cellular components according to environmental conditions through the regulation of a complex gene expression network.
- Receptor-binding complex
-
A phage-encoded structural protein complex that is essential for the adsorption of the phage to the bacterial cell. In tailed phages, this complex is located at the extremity of the tail.
- O antigen
-
The outer-most part of the lipopolysaccharide on the bacterial outer membrane, containing a repetitive glycan polymer. A great diversity is observed in the structure of E. coli O antigens, and they are good targets for serotyping methods and phages.
- K antigen
-
Polysaccharide in the bacterial capsule.
- Restriction enzyme
-
An endonuclease protects the bacterial cell against infection by cleaving foreign DNA at specific sites. These enzymes are generally coupled with a cognate DNA methylase, which modifies and protects the host DNA.
- Two-component system
-
A biological mechanism necessitating the presence of two enzymes to be functional.
- Cryptic genetic element
-
An incomplete or defective prophage that is unable to excise from the host genome and multiply as a result of host genome evolution. Such prophages provide a pool of phage genes that can be tapped into by an incoming virulent phage.
Rights and permissions
About this article
Cite this article
Labrie, S., Samson, J. & Moineau, S. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8, 317–327 (2010). https://doi.org/10.1038/nrmicro2315
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2315
This article is cited by
-
Positive and negative aspects of bacteriophages and their immense role in the food chain
npj Science of Food (2024)
-
Isolation, characterization, therapeutic potency, and genomic analysis of a novel bacteriophage vB_KshKPC-M against carbapenemase-producing Klebsiella pneumoniae strains (CRKP) isolated from Ventilator-associated pneumoniae (VAP) infection of COVID-19 patients
Annals of Clinical Microbiology and Antimicrobials (2023)
-
Isolation and characterization of bacteriophages for combating multidrug-resistant Listeria monocytogenes from dairy cattle farms in conjugation with silver nanoparticles
BMC Microbiology (2023)
-
Challenges of bacteriophages application in controlling bacterial plant diseases and how to overcome them
Journal of Genetic Engineering and Biotechnology (2023)
-
Bacteria can shed a layer when phages turn up the heat
Nature Microbiology (2023)