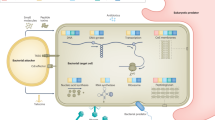
Key Points
-
Streptococci are often carried on mucosal surfaces and skin without causing disease.
-
Streptococci can cause diseases that range from dental caries to necrotizing fasciitis. The type of disease caused depends on the virulence factors produced by the bacterium, as well as host susceptibility.
-
Streptococcus mutans causes dental caries; this requires the ability of this organism to form biofilms and to produce acid on the tooth surface.
-
Streptococcus agalactiae — group B streptococcus (GBS) — can cause neonatal meningitis. The organism is usually acquired by the neonate during passage through the birth canal of a colonized mother. Key virulence factors are the capsule, surface proteins and a haemolysin.
-
Streptococcus pyogenes — group A streptococcus (GAS) — can cause a wide range of diseases. Toxic-shock syndrome that is caused by this organism is mediated by the production of superantigens. The organism makes a capsule, a wide range of toxins and enzymes, and molecules that interfere with innate immunity.
-
Streptococcus pneumoniae (the pneumococcus) can cause pneumonia and meningitis. The organism induces overactivation of the inflammatory response, and disease symptoms partly reflect inappropriate production of host immune mediators. Key virulence factors are the capsule, surface proteins and pneumolysin.
-
Complete genome sequences are available for nine strains of these four species of streptococci (one for S. mutans, two for GBS, four for GAS and two for pneumococcus). Analysis of the genes present in all four species shows that the highly conserved genes are concerned with core function, such as nucleic-acid metabolism and protein synthesis. There is also significant variation in gene content, even between strains of the same species.
-
Simultaneous analysis of expression of all genes in the genome is now possible using microarray technology. It is also possible to examine the expression of genes in organisms that cause infection in model systems or in human disease. Such studies are beginning to allow an understanding of the detailed interactions that occur between pathogens and their hosts.
Abstract
The development of bacterial disease has been likened to a 'molecular arms race', in which the host tries to eliminate the bacteria, while the bacteria try to survive in the host. Although most bacteria do not cause disease, some cause serious human infection in a large proportion of encounters. Between these two extremes are bacteria that can coexist with humans in a carriage state but, under appropriate circumstances, cause disease. The streptococci exemplify this group of organisms, and by studying them we can begin to address why bacteria cause such a wide spectrum of disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Loesche, W. J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50, 353–380 (1986).
Quivey, R. G., Kuhnert, W. L. & Hahn, K. Genetics of acid adaptation in oral streptococci. Crit. Rev. Oral Biol. Med. 12, 301–314 (2001).
Bhagwat, S. P., Nary, J. & Burne, R. A. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205, 225–230 (2001).
Jenkinson, H. F. & Demuth, D. R. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23, 183–190 (1997).
Ma, J. K. et al. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect. Immun. 58, 3407–3414 (1990).
Lee, S. F. & Boran, T. L. Roles of sortase in surface expression of the major protein adhesin P1, saliva-induced aggregation and adherence, and cariogenicity of Streptococcus mutans. Infect. Immun. 71, 676–681 (2003). Describes the use of genetic manipulation in combination with an animal model to show the importance of the cell-surface anchoring of proteins in the ability of S. mutans to cause caries.
Kuramitsu, H. K. Virulence factors of mutans streptococci: role of molecular genetics. Crit. Rev. Oral Biol. Med. 4, 159–176 (1993).
Hazlett, K. R. O., Michalek, S. M. & Banas, J. A. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect. Immun. 66, 2180–2185 (1998).
Munro, C., Michalek, S. M. & Macrina, F. L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 59, 2316–2323 (1991).
Yamashita, Y., Bowen, W. H., Burne, R. A. & Kuramitsu, H. K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 61, 3811–3817 (1993).
Idone, V. et al. Effect of an orphan response regulator on Streptococcus mutans sucrose-dependent adherence and cariogenesis. Infect. Immun. 71, 4351–4360 (2003).
Russell, M., Harrington, D. & Russell, R. Identity of Streptococcus mutans surface protein antigen III and wall-associated protein antigen A. Infect. Immun. 63, 733–735 (1995).
Kitten, T., Munro, C. L., Michalek, S. M. & Macrina, F. L. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68, 4441–4451 (2000).
Haas, W. & Banas, J. A. Ligand-binding properties of the carboxyl-terminal repeat domain of Streptococcus mutans glucan-binding protein A. J. Bacteriol. 182, 728–733 (2000).
Sato, Y., Yamamoto, Y. & Kizaki, H. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 65, 668–675 (1997).
Holmes, A. R. et al. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 41, 1395–1408 (2001).
Dintilhac, A., Alloing, G., Granadel, C. & Claverys, J. P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25, 727–739 (1997).
Berry, A. M. & Paton, J. C. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64, 5255–5262 (1996).
Marra, A. et al. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148, 1483–1491 (2002).
Romero-Steiner, S. et al. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10, 246–251 (2003).
Ajdić, D. I. et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl Acad. Sci. USA 99, 14434–14439 (2002). Contains a description of the S. mutans genome and an analysis of metabolic and regulatory aspects revealed by the genomic sequence.
Schuchat, A. Group B streptococcal disease: from trials and tribulations to triumph and trepidation. Clin. Infect. Dis. 33, 751–756 (2001).
Schuchat, A. Epidemiology of Group B streptococcal disease in the United States: shifting paradigms. Clin. Microbiol. Rev. 11, 497–513 (1998).
Rubens, C. E. et al. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 60, 5157–5163 (1992).
Gibson, R. L. et al. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect. Immun. 61, 478–485 (1993).
Jones, A. L., Knoll, K. M. & Rubens, C. E. Identification of Streptococcus agalactiae virulence genes in the neonatal rat sepsis model using signature-tagged mutagenesis. Mol. Microbiol. 37, 1444–1455 (2000).
Marques, M. B., Kasper, D. L., Pangburn, M. K. & Wessels, M. R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 60, 3986–3993 (1992).
Spellerberg, B. et al. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67, 871–878 (1999).
Li, J. et al. Inactivation of the α C protein antigen gene, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B Streptococcus. Proc. Natl Acad. Sci. USA 94, 13251–13256 (1997).
Schubert, A. et al. A fibrinogen receptor from group B Streptococcus. Mol. Microbiol. 46, 557–569 (2002).
Glaser, P. et al. Genome sequence of Streptococcus agalactiae. Mol. Microbiol. 45, 1499–1513 (2002). This paper and reference 147 describe the genome sequence of GBS, giving details of genomic structure, comparative genomics and metabolic insights from the two strains sequenced.
Chmouryguina, I., Suvorov, A., Ferrieri, P. & Cleary, P. Conservation of the C5a peptidase genes in group A and B streptococci. Infect. Immun. 64, 2387–2390 (1996).
Pritzlaff, C. A. et al. Genetic basis for the β-haemolytic/cytolytic activity of group B Streptococcus. Mol. Microbiol. 39, 236–248 (2001).
Nizet, V. et al. Group B streptococcal β-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64, 3818–3826 (1996).
Gibson, R. L., Nizet, V. & Rubens, C. E. Group B streptococcal β-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr. Res. 45, 626–634 (1999).
Nizet, V. et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65, 5074–5081 (1997).
Ring, A. et al. Group B streptococcal β-hemolysin induces nitric oxide production in murine macrophages. J. Infect. Dis. 182, 150–157 (2000).
Henneke, P. et al. Cellular activation, phagocytosis, and bactericidal activity against Group B Streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 169, 3970–3977 (2002).
Doran, K. S. et al. Group B streptococcal β-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185, 196–203 (2002).
Nizet, V., Gibson, R. L. & Rubens, C. E. The role of group B streptococci β-hemolysin expression in newborn lung injury. Adv. Exp. Med. Biol. 418, 627–630 (1997).
Puliti, M. et al. Severity of group B streptococcal arthritis is correlated with β-hemolysin expression. J. Infect. Dis. 182, 824–832 (2000).
Ring, A. et al. Group B streptococcal β-hemolysin induces mortality and liver injury in experimental sepsis. J. Infect. Dis. 185, 1745–1753 (2002).
Bisno, A. L., Brito, M. O. & Collins, C. M. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3, 191–200 (2003). A detailed review of the virulence factors of S. pyogenes.
Courtney, H. S., Hasty, D. L. & Dale, J. B. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann. Med. 34, 77–87 (2002).
Hasty, D. L., Ofek, I., Courtney, H. S. & Doyle, R. J. Multiple adhesins of streptococci. Infect. Immun. 60, 2147–2152 (1992).
Okada, N., Liszewski, M., Atkinson, J. & Caparon, M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A Streptococcus. Proc. Natl Acad. Sci. USA 92, 2489–2493 (1995).
Talay, S. R. et al. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 60, 3837–3844 (1992).
Hanski, E., Horwitz, P. A. & Caparon, M. G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect. Immun. 60, 5119–5125 (1992).
Kreikemeyer, B., Talay, S. R. & Chhatwal, G. S. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17, 137–145 (1995).
Courtney, H., Dale, J. & Hasty, D. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect. Immun. 64, 2415–2419 (1996).
Jaffe, J., Natanson-Yaron, S., Caparon, M. G. & Hanski, E. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21, 373–384 (1996).
Rocha, C. L. & Fischetti, V. A. Identification and characterization of a novel fibronectin-binding protein on the surface of Group A streptococci. Infect. Immun. 67, 2720–2728 (1999).
Schrager, H. M. et al. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Invest. 101, 1708–1716 (1998).
Cywes, C. & Wessels, M. R. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414, 648–652 (2001). Describes the role of the hyaluronic acid capsule of S. pyogenes in the interaction with CD44 on host cells and the importance of the subsequent signalling events
Horstmann, R. D., Sievertsen, H. J., Knobloch, J. & Fischetti, V. A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl Acad. Sci. USA 85, 1657–1661 (1988).
Johnsson, E. et al. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161, 4894–4901 (1998).
Whitnack, E. & Beachey, E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J. Exp. Med. 162, 1983–1997 (1985).
Whitnack, E., Dale, J. B. & Beachey, E. H. Common protective antigens of group A streptococcal M proteins masked by fibrinogen. J. Exp. Med. 159, 1201–1212 (1984).
Berge, A., Kihlberg, B. -M., Sjoholm, A. G. & Bjorck, L. Streptococcal protein H forms soluble complement-activating complexes with IgG, but inhibits complement activation by IgG-coated targets. J. Biol. Chem. 272, 20774–20781 (1997).
Collin, M. et al. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect. Immun. 70, 6646–6651 (2002).
Cleary, P. P. et al. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 60, 5219–5223 (1992).
Dale, J., Washburn, R., Marques, M. & Wessels, M. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect. Immun. 64, 1495–1501 (1996).
Lei, B. et al. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nature Med. 7, 1298–1305 (2001). Shows the importance of Mac in the ability of S. pyogenes to interfere with complement activation and phagocytosis. A mechanism is proposed for Mac function.
Zhou, M. J. & Brown, E. J. CR3 (Mac-1, αMβ2, CD11b/CD18) and Fc-γRIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc-γRIII and tyrosine phosphorylation. J. Cell Biol. 125, 1407–1416 (1994).
Åkesson, P., Sjöholm, A. G. & Björck, L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271, 1081–1088 (1996).
Fernie-King, B. A. et al. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology 103, 390–398 (2001).
Fernie-King, B. A., Seilly, D. J., Davies, A. & Lachmann, P. J. Streptococcal inhibitor of complement inhibits two additional components of the mucosal innate immune system: secretory leukocyte proteinase inhibitor and lysozyme. Infect. Immun. 70, 4908–4916 (2002).
Frick, I. -M. et al. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 278, 16561–16566 (2003).
Madden, J. C., Ruiz, N. & Caparon, M. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in Gram-positive bacteria. Cell 104, 143–152.
Limbago, B., Penumalli, V., Weinrick, B. & Scott, J. R. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68, 6384–6390 (2000).
Fontaine, M. C., Lee, J. J. & Kehoe, M. A. Combined contributions of streptolysin O and streptolysin S to virulence of serotype M5 Streptococcus pyogenes strain Manfredo. Infect. Immun. 71, 3857–3865 (2003).
Ofek, I., Zafriri, D., Goldhar, J. & Eisenstein, B. I. Inability of toxin inhibitors to neutralize enhanced toxicity caused by bacteria adherent to tissue culture cells. Infect. Immun. 58, 3737–3742 (1990).
Betschel, S. D. et al. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect. Immun. 66, 1671–1679 (1998).
Marrack, P. & Kappler, J. The staphylococcal enterotoxins and their relatives. Science 248, 1066 (1990).
Christner, R. et al. Identification of key gene products required for acquisition of plasmin-like enzymatic activity by group A streptococci. J. Infect. Dis. 175, 1115–1120 (1997).
Rasmussen, M., Muller, H. -P. & Bjorck, L. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding α2-macroglobulin. J. Biol. Chem. 274, 15336–15344 (1999).
Toppel, A. W. et al. Contribution of protein G-related α2-macroglobulin-binding protein to bacterial virulence in a mouse skin model of group A streptococcal infection. J. Infect. Dis. 187, 1694–1703 (2003).
Lukomski, S. et al. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 66, 771–776 (1998).
Saouda, M., Wu, W., Conran, P. & Boyle, M. D. Streptococcal pyrogenic exotoxin B enhances tissue damage initiated by other Streptococcus pyogenes products. J. Infect. Dis. 184, 723–731 (2001).
Collin, M. & Olsen, A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 69, 7187–7189 (2001).
Jonsson, S. et al. Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J. Infect. Dis. 152, 4–13 (1985).
Polissi, A. et al. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect. Immun. 66, 5620–5629 (1998).
Hava, D. & Camilli, A. Large-scale identificationof serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45, 1389–1406 (2002).
Lau, G. W. et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40, 555–571 (2001).
Cundell, D. R. et al. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377, 435–438 (1995).
Tuomanen, E. et al. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151, 859–868 (1985).
Canvin, J. R. et al. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type-2 pneumococcus. J. Infect. Dis. 2, 119–123 (1995).
Alcantara, R. B., Preheim, L. C. & Gentry, M. J. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 67, 2862–2866 (1999).
Benton, K. A., Everson, M. P. & Briles, D. E. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63, 448–455 (1995).
Berry, A. M. et al. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57, 2037–2042 (1989).
Braun, J. S. et al. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Invest. 109, 19–27 (2002). Gives details of the role and mechanism of pneumolysin and hydrogen peroxide in inducing brain damage during pneumococcal meninigitis.
Comis, S. D. et al. Cytotoxic effects on hair cells of guinea pig cochlea produced by pneumolysin, the thiol activated toxin of Streptococcus pneumoniae. Acta Otolaryngol. 113, 152–159 (1993).
Johnson, M. K. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr. Eye Res. 9, 1107–1114 (1979).
Houldsworth, S., Andrew, P. W. & Mitchell, T. J. Pneumolysin stimulates production of tumor necrosis factor α and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62, 1501–1503 (1994).
Braun, J. S. et al. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 67, 3750–3756 (1999).
Cockeran, R. et al. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J. Infect. Dis. 186, 562–565 (2002).
Cockeran, R. et al. Pneumolysin potentiates production of prostaglandin E-2 and leukotriene B-4 by human neutrophils. Infect. Immun. 69, 3494–3496 (2001).
Rubins, J. B., Mitchell, T. J., Andrew, P. W. & Niewoehner, D. E. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect. Immun. 62, 3829–3836 (1994).
Rubins, J. B. et al. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect. Immun. 61, 1352–1358 (1993).
Rubins, J. B., Duane, P. G., Charboneau, D. & Janoff, E. N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect. Immun. 60, 1740–1746 (1992).
Steinfort, C. et al. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect. Immun. 57, 2006–2013 (1989).
Paton, J. C. & Ferrante, A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41, 1212–1216 (1983).
Ferrante, A., Rowan-Kelly, B. & Paton, J. C. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46, 585–589 (1984).
Paton, J. C., Rowan-Kelly, B. & Ferrante, A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43, 1085–1087 (1984).
Rubins, J. B. et al. Distinct role for pneumolysin's cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 153, 1339–1346 (1996).
Hirst, R. A. et al. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect. Immun. 68, 1557–1562 (2000).
Stringaris, A. K. et al. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol. Dis. 11, 355–368 (2002).
Malley, R. et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl Acad. Sci. USA 100, 1966–1971 (2003).
Crain, M. J. et al. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58, 3293–3299 (1990).
McDaniel, L. S. et al. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165, 381–394 (1987).
Neeleman, C. et al. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67, 4517–4524 (1999).
Tu, A. T. et al. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67, 4720–4724 (1999).
Hammerschmidt, S., Bethe, G., Remane, P. H. & Chhatwal, G. S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67, 1683–1687 (1999).
Rosenow, C. et al. Contribution of a novel choline binding protein to adherence, colonization, and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25, 819–829 (1997).
Smith, B. L. & Hostetter, M. K. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 182, 497–508 (2000).
Janulczyk, R. et al. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275, 37257–37263 (2000).
Dave, S., Brooks-Walter, A., Pangburn, M. K. & McDaniel, L. S. PspC, a pneumococcal surface protein, binds human Factor H. Infect. Immun. 69, 3435–3437 (2001).
Madsen, M. et al. A pneumococcal protein that elicits interleukin-8 from pulmonary epithelial cells. J. Infect. Dis. 181, 1330–1336 (2000).
Zhang, J. R. et al. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102, 827–837 (2000).
Berry, A. M., Lock, R. A., Hansman, D. & Paton, J. C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 57, 2324–2330 (1989).
Gosink, K. K. et al. Role of novel choline binding proteins in virulence of Streptococcus pneumoniae. Infect. Immun. 68, 5690–5695 (2000).
Tettelin, H. et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293, 498–506 (2001). Highlights the differences between the stains and shows the ability of the organism to transport many sugars.
Humphrey, J. H. Hyaluronidase production by pneumococci. J. Pathol. Bacteriol. 55, 273–275 (1948).
Berry, A. M. & Paton, J. C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68, 133–140 (2000).
Camara, M., Boulnois, G. J., Andrew, P. W. & Mitchell, T. J. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 62, 3688–3695 (1994).
Tong, H. H., Blue, L. E., James, M. A. & DeMaria, T. F. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68, 921–924 (2000).
Winter, A. J. et al. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65, 4411–4418 (1997).
Kharat, A. S. & Tomasz, A. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect. Immun. 71, 2758–2765 (2003).
Lawrence, M. C. et al. The crytal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6, 1553–1561 (1998).
Tseng, H. -J., McEwan, A. G., Paton, J. C. & Jennings, M. P. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70, 1635–1639 (2002).
Bergmann, S. et al. Identification of a novel plasmin(ogen)-binding motif in surface displayed α-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49, 411–423 (2003).
Yesilkaya, H. et al. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68, 2819–2826 (2000).
Auzat, I. et al. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34, 1018–1028 (1999).
Duane, P. G., Rubins, J. B., Weisel, H. R. & Janoff, E. N. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect. Immun. 61, 4392–4397 (1993).
Brown, J. S., Gilliland, S. M. & Holden, D. W. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40, 572–585 (2001).
Peterson, J. D. et al. The Comprehensive Microbial Resource. Nucleic Acids Res. 29, 123–125 (2001).
Smoot, L. M. et al. Characterization of two novel pyrogenic toxin superantigens made by an acute rheumatic fever clone of Streptococcus pyogenes associated with multiple disease outbreaks. Infect. Immun. 70, 7095–7104 (2002).
Lei, B. et al. Opsonophagocytosis-inhibiting Mac protein of group A Streptococcus: identification and characteristics of two genetic complexes. Infect. Immun. 70, 6880–6890 (2002).
Banks, D. J., Beres, S. B. & Musser, J. M. The fundamental contribution of phages to GAS evolution, genome diversification and strain emergence. Trends Microbiol. 10, 515–521 (2002).
Smoot, J. C. et al. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl Acad. Sci. USA 99, 4668–4673 (2002). This paper and references 148, 149 and 150 describe the genome sequences of four strains of GAS. These studies describe the genomic organization of the strains, microarray analysis of strains associated with acute rheumatic fever, and provide an insight into the role of phages and genomic rearrangements in virulence.
Voyich, J. M. et al. Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proc. Natl Acad. Sci. USA 100, 1996–2001 (2003). Analysis of genome-wide gene-expression changes on interaction of GAS with human polymorphonuclear cells. Confirmation of the role of a two-component system in virulence and that this system is upregulated during human infection.
Graham, M. R. et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl Acad. Sci. USA 99, 13855–13860 (2002). Global expression profiling in GAS using microarrays to examine the control of virulence gene expression.
Virtaneva, K. et al. Group A Streptococcus gene expression in humans and cynomolgus macaques with acute pharyngitis. Infect. Immun. 71, 2199–2207 (2003). Examination of gene expression in GAS when associated with pharyngitis. Use of human samples and an animal model to measure gene expression during the disease process.
Kihlberg, B. M. et al. Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb. Pathog. 19, 299–315 (1995).
Fournier, B., Klier, A. & Rapoport, G. The two-component system ArlS–ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 41, 247–261 (2001).
Tettelin, H. et al. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl Acad. Sci. USA 99, 12391–12396 (2002).
Beres, S. B. et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl Acad. Sci. USA 99, 10078–10083 (2002).
Ferretti, J. J. et al. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl Acad. Sci. USA 98, 4658–4663 (2001).
Nakagawa, I. et al. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 13, 1042–1055 (2003).
Hoskins, J. et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183, 5709–5717 (2001).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez
FURTHER INFORMATION
Comprehensive Microbial Resource
Infectious disease information
Glossary
- ENDOCARDITIS
-
Inflammation of the lining of the heart and its valves.
- TWO-COMPONENT SIGNAL-TRANSDUCTION PATHWAY
-
A signal-transduction system that consists of a sensor protein that senses and responds to an external signal, and which acts on a response-regulator protein that transmits the signal to other components of the cell.
- LECTIN
-
A cell-agglutinating protein of non-immune origin, which binds carbohydrates without modifying them.
- PASSIVE IMMUNIZATION
-
The induction of immunity by the transfer of immunoglobulins or T cells.
- SORTASE
-
An enzyme that links proteins to the cell wall.
- HYDROXYAPATITE
-
The main mineral component of teeth.
- GLUCOSYLTRANSFERASE
-
Any hexosyltransferase enzyme for which the glycosyl group transferred is glucosyl.
- GERM-FREE RATS
-
Rats bred and maintained in such conditions that they contain no microorganisms.
- SIGNATURE-TAGGED MUTAGENESIS
-
A negative selection method for the identification of virulence genes. The technique involves mutation of microbial genes by random insertion of a tagged transposon. After growth of a mixed population in a host, mutated genes that are absent and hence required for virulence are identified with the help of the tag.
- OPSONOPHAGOCYTOSIS
-
Increased uptake of bacteria by host cells due to binding of antibody or complement.
- β-HAEMOLYSIS
-
Zone of clearing around bacterial colonies grown on blood agar, caused by lysis of red blood cells.
- HAEMOLYSIN
-
Material that lyses red blood cells.
- ENDOGLYCOSIDASE
-
An enzyme that hydrolyses non-terminal glycosidic linkages in oligosaccharides or polysaccharides.
- MEMBRANE-ATTACK COMPLEX
-
A collection of late complement components that damage the cell membrane.
- PYOGENIC EXOTOXINS
-
Toxins that induce fever.
- SUPERANTIGEN
-
A protein that activates T cells non-specifically.
- STREPTOCOCCAL TOXIC-SHOCK SYNDROME
-
A disease associated with decreased blood pressure and multi-organ failure.
- EPENDYMAL CILIA
-
Specialized ciliated cells that line the ventricles of the brain.
- ENOLASE
-
Also known as phosphopyruvate hydratase. An enzyme that catalyses the conversion of 2-phospho-D-glycerate to phosphoenolpyruvate and water.
Rights and permissions
About this article
Cite this article
Mitchell, T. The pathogenesis of streptococcal infections: from Tooth decay to meningitis. Nat Rev Microbiol 1, 219–230 (2003). https://doi.org/10.1038/nrmicro771
Issue Date:
DOI: https://doi.org/10.1038/nrmicro771
This article is cited by
-
Inhibitory effects of Bacillus velezensis ID-A01 supernatant against Streptococcus mutans
BMC Microbiology (2023)
-
SIgA structures bound to Streptococcus pyogenes M4 and human CD89 provide insights into host-pathogen interactions
Nature Communications (2023)
-
Analysis of virulence factors and antibiotic resistance genes in group B streptococcus from clinical samples
BMC Infectious Diseases (2021)
-
Phenotypic changes in group B streptococci grown in the presence of the polyols, erythritol, sorbitol and mannitol
BMC Microbiology (2021)
-
Mucin O-glycans suppress quorum-sensing pathways and genetic transformation in Streptococcus mutans
Nature Microbiology (2021)