Key Points
-
Type IV pili are strong, flexible filaments with varied roles in bacterial pathogenicity. Pilin structures and models are integrated here in a comprehensive review of the molecular basis of the assembly and multi-functionality of these filaments.
-
Type IV pili are grouped together as a class on the basis of sequence similarity of their major subunit protein, pilin, which has an unusual N-methylated amino terminus, a conserved hydrophobic N-terminal 25 residues, and a carboxy-terminal disulphide bond and can be divided into two subclasses, type IVa and type IVb. Type IVa pili occur on a wide range of Gram-negative bacteria, but so far, type IVb are restricted to human enteric bacteria.
-
The authors succinctly draw together the challenges that structural studies of type IV pili have presented, then review these structures and highlight specific structural features that are related to the assembly and function of type IV pili.
-
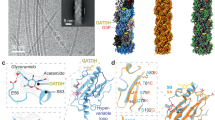
The authors present structure-based models for the type IVb toxin-co-regulated pilus (TCP) from Vibrio cholerae and the type IVa PAK pilus from Pseudomonas aeruginosa. These assembly models are surprisingly similar despite substantial differences in protein sequence, size and structure between the pilin subunits, and lead to a generalized assembly model for type IV pili. The PAK pilus model is presented here for the first time.
-
There are striking similarities between type IV pilus, archaeal flagellar and the type II secretion systems of bacteria. Archaeal flagella are required for motility, and type II secretion systems encode the machinery to export enzymes and toxins. How type IV pilus biogenesis and structure could help to generate hypotheses about the function of the archaeal flagellum and type II secretion systems are discussed.
-
The authors elaborate on the multiple functions of the type IV pili, which include adhesion to surfaces, immune escape, surface motility, microcolony formation, transformation and phage transduction, signalling to eukaryotic cells, apoptosis and complement inhibition, and in each case describe how the knowledge of type IV pilus structure and assembly can help us to understand these processes.
Abstract
Type IV pili are remarkably strong, flexible filaments with varied roles in bacterial pathogenicity. All Gram-negative bacterial surfaces have type IV pili, which are polymeric assemblies of the protein pilin that evoke the host immune response and are potential drug and vaccine targets. Pilin structures that have been solved using X-ray crystallography and nuclear magnetic resonance, together with models for pilus architectures inferred from electron microscopy, fibre diffraction and computation, have established a molecular basis for assembly and multi-functionality, with implications for therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Levine, M. M. et al. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152, 550–559 (1985).
Herrington, D. A. et al. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168, 1487–1492 (1988).
Tacket, C. O. et al. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect. Immun. 66, 692–695 (1998).
Bieber, D. et al. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280, 2114–2118 (1998).
Maier, B., Potter, L., So, M., Seifert, H. S. & Sheetz, M. P. Single pilus motor forces exceed 100 pN. Proc. Natl Acad. Sci. USA 99, 16012–16017 (2002).
Merz, A. J., So, M. & Sheetz, M. P. Pilus retraction powers bacterial twitching motility. Nature 407, 98–102 (2000). The authors use laser tweezers to show that N. gonorrhoeae type IV pili tether the cells to a quartz surface and retract with a force that can exceed 80 pN, pulling cells forward, and that this process requires functional PilT.
Bragg, S. L. The Development of X-ray Analysis (eds Phillips, D. C. & Lipson, H.) (G. Bell, London, 1975).
Schutt, C. E., Myslik, J. C., Rozycki, M. D., Goonesekere, N. C. & Lindberg, U. The structure of crystalline profilin-β-actin. Nature 365, 810–816 (1993).
Kabsch, W., Mannherz, H. G. & Suck, D. Three-dimensional structure of the complex of actin and DNase I at 4.5 Å resolution. EMBO J. 4, 2113–2118 (1985).
McLaughlin, P. J., Gooch, J. T., Mannherz, H. G. & Weeds, A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature 364, 685–692 (1993).
Robinson, R. C. et al. Domain movement in gelsolin: a calcium-activated switch. Science 286, 1939–1942 (1999).
Otterbein, L. R., Graceffa, P. & Dominguez, R. The crystal structure of uncomplexed actin in the ADP state. Science 293, 708–711 (2001).
Rayment, I. et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50–58 (1993).
Samatey, F. A. et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410, 331–337 (2001).
Sauer, F. G. et al. Structural basis of chaperone function and pilus biogenesis. Science 285, 1058–1061 (1999).
Craig, L. et al. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell. 11, 1139–1150 (2003). Describes the first type IVb pilin structure for V. cholerae TcpA, which reveals a new protein fold, together with a full-length PAK pilin structure and a model for V. cholerae pilus assembly that is applicable to all type IV pili.
Parge, H. E. et al. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 378, 32–38 (1995). The first structure of a type IV pilin reveals a ladle-shaped molecule with a 52-residue long N-terminal α-helix, an αβ-roll fold, a hypervariable β-hairpin and a covalently linked phosphate and disaccharide for GC pilin. A model for pilus assembly is presented based on symmetry of the PAK pilus filament.
Hazes, B., Sastry, P. A., Hayakawa, K., Read, R. J. & Irvin, R. T. Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J. Mol. Biol. 299, 1005–1017 (2000). This first structure of the PAK pilin globular head domain reveals a similar fold to GC pilin and a receptor-binding loop that presents a surface dominated by main chain atoms.
Keizer, D. W. et al. Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J. Biol. Chem. 276, 24186–24193 (2001). NMR solution structure of the soluble domain of P. aeruginosa K122-4 pilin showing an unusually large angle between the N-terminal α-helix and β-sheet compared with GC and PAK pilin. A model for K122-4 pilus assembly is also presented.
Yonekura, K., Maki-Yonekura, S. & Namba, K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424, 643–650 (2003).
Li, H., DeRosier, D., Nicholson, W., Nogales, E. & Downing, K. Microtubule structure at 8 Å resolution. Structure 10, 1317 (2002).
Zhu, Y., Carragher, B., Kriegman, D. J., Milligan, R. A. & Potter, C. S. Automated identification of filaments in cryo-electron microscopy images. J. Struct. Biol. 135, 302–312 (2001).
Pauling, L. & Corey, R. B. Two hydrogen-bonded spiral configurations of the polypeptide chain. J. Am. Chem. Soc. 72, 534 (1950).
Franklin, R. & Gosling, R. G. Molecular configuration in sodium thymonucleate. Nature 171, 740–741 (1953).
Watson, J. D. & Crick, F. H. C. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171, 737–738 (1953).
Kishchenko, G., Batliwala, H. & Makowski, L. Structure of a foreign peptide displayed on the surface of bacteriophage M13. J. Mol. Biol. 241, 208–213 (1994).
Bryan, R. K., Bansal, M., Folkhard, W., Nave, C. & Marvin, D. A. Maximum-entropy calculation of the electron density at 4 Å resolution of Pf1 filamentous bacteriophage. Proc. Natl Acad. Sci. USA 80, 4728–4731 (1983).
Peterson, C., Winter, W. T., Dalack, G. W. & Day, L. A. Structure of the filamentous bacteriophage, Pf3, by X-ray fiber diffraction. J. Mol. Biol. 162, 877–881 (1982).
Marvin, D. A., Nadassy, K., Welsh, L. C. & Forest, K. Type-4 bacterial pili: molecular models and their simulated diffraction patterns. Fibre Diffract. Rev. 11, 87–94 (2003). X-ray fibre diffraction data for GC and PAK pili indicate differences in their packing arrangements.
Marvin, D. A. & Folkhard, W. Structure of F-pili: reassessment of the symmetry. J. Mol. Biol. 191, 299–300 (1986).
Serpell, L. C., Berriman, J., Jakes, R., Goedert, M. & Crowther, R. A. Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc. Natl Acad. Sci. USA 97, 4897–4902 (2000).
Perutz, M. F., Finch, J. T., Berriman, J. & Lesk, A. Amyloid fibers are water-filled nanotubes. Proc. Natl Acad. Sci. USA 99, 5591–5595 (2002).
Morozova-Roche, L. A. et al. Amyloid fibril formation and seeding by wild-type human lysozyme and its disease-related mutational variants. J. Struct. Biol. 130, 339–351 (2000).
Inouye, H. et al. Analysis of X-ray diffraction patterns from amyloid of biopsied vitreous humor and kidney of transthyretin (TTR) Met30 familial amyloidotic polyneuropathy (FAP) patients: axially arrayed TTR monomers constitute the protofilament. Amyloid 5, 163–174 (1998).
Folkhard, W., Marvin, D. A., Watts, T. H. & Paranchych, W. Structure of polar pili from Pseudomonas aeruginosa strains K and O. J. Mol. Biol. 149, 79–93 (1981). First X-ray fibre diffraction analysis of PAK and PAO pili defining their helical symmetry.
Swanson, J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137, 571–589 (1973).
Potts, W. J. & Saunders, J. R. Nucleotide sequence of the structural gene for class I pilin from Neisseria meningitidis: homologies with the pilE locus of Neisseria gonorrhoeae. Mol. Microbiol. 2, 647–653 (1988).
Meyer, T. F., Billyard, E., Haas, R., Storzbach, S. & So, M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc. Natl Acad. Sci. USA 81, 6110–6114 (1984).
Bradley, D. E. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can. J. Microbiol. 26, 146–154 (1980).
Woods, D. E., Straus, D. C., Johanson, W. G. Jr, Berry, V. K. & Bass, J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect. Immun. 29, 1146–1151 (1980).
Johnson, K., Parker, M. L. & Lory, S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J. Biol. Chem. 261, 15703–15708 (1986).
McKern, N. M., Stewart, D. J. & Strike, P. M. Amino acid sequences of pilins from serologically distinct strains of Bacteroides nodosus. J. Protein Chem. 7, 157–164 (1988).
Marrs, C. F. et al. Cloning and sequencing of a Moraxella bovis pilin gene. J. Bacteriol. 163, 132–139 (1985).
Tonjum, T., Marrs, C. F., Rozsa, F. & Bovre, K. The type 4 pilin of Moraxella nonliquefaciens exhibits unique similarities with the pilins of Neisseria gonorrhoeae and Dichelobacter (Bacteroides) nodosus. J. Gen. Microbiol. 137, 2483–2490 (1991).
Tonjum, T., Weir, S., Bovre, K., Progulske-Fox, A. & Marrs, C. F. Sequence divergence in two tandemly located pilin genes of Eikenella corrodens. Infect. Immun. 61, 1909–1916 (1993).
Dorr, J., Hurek, T. & Reinhold-Hurek, B. Type IV pili are involved in plant–microbe and fungus–microbe interactions. Mol. Microbiol. 30, 7–17 (1998).
Rendulic, S. et al. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303, 689–692 (2004).
Faast, R., Ogierman, M. A., Stroeher, U. H. & Manning, P. A. Nucleotide sequence of the structural gene, tcpA, for a major pilin subunit of Vibrio cholerae. Gene 85, 227–231 (1989).
Shaw, C. E. & Taylor, R. K. Vibrio cholerae O395 tcpA pilin gene sequence and comparison of predicted protein structural features to those of type 4 pilins. Infect. Immun. 58, 3042–3049 (1990).
Zhang, X. L. et al. Salmonella enterica serovar Typhi uses type IVB pili to enter human intestinal epithelial cells. Infect. Immun. 68, 3067–3073 (2000).
Donnenberg, M. S., Giron, J. A., Nataro, J. P. & Kaper, J. B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6, 3427–3437 (1992).
Giron, J. A., Ho, A. S. & Schoolnik, G. K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254, 710–713 (1991).
Taniguchi, T., Fujino, Y., Yamamoto, K., Miwatani, T. & Honda, T. Sequencing of the gene encoding the major pilin of pilus colonization factor antigen III (CFA/III) of human enterotoxigenic Escherichia coli and evidence that CFA/III is related to type IV pili. Infect. Immun. 63, 724–728 (1995).
Giron, J. A., Levine, M. M. & Kaper, J. B. Longus: a long pilus ultrastructure produced by human enterotoxigenic Escherichia coli. Mol. Microbiol. 12, 71–82 (1994).
Pasloske, B. L., Scraba, D. G. & Paranchych, W. Assembly of mutant pilins in Pseudomonas aeruginosa: formation of pili composed of heterologous subunits. J. Bacteriol. 171, 2142–2147 (1989).
Strom, M. S. & Lory, S. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J. Biol. Chem. 266, 1656–1664 (1991).
Macdonald, D. L., Pasloske, B. L. & Paranchych, W. Mutations in the fifth position glutamate in Pseudomonas aeruginosa pilin affect the transmethylation of the N-terminal phenylalanine. Can. J. Microbiol. 39, 500–505 (1993).
Forest, K. T. & Tainer, J. A. Type-4 pilus-structure: outside to inside and top to bottom — a minireview. Gene 192, 165–169 (1997).
Marceau, M., Forest, K., Beretti, J. L., Tainer, J. & Nassif, X. Consequences of the loss of O-linked glycosylation of meningococcal type IV pilin on piliation and pilus-mediated adhesion. Mol. Microbiol. 27, 705–715 (1998). The authors identify the MC pilin glycosylation site at Ser63 and show that glycosylation does not have an important role in pilus assembly or adhesion but is required for expression of S pilin.
Forest, K. T., Dunham, S. A., Koomey, M. & Tainer, J. A. Crystallographic structure reveals phosphorylated pilin from Neisseria: phosphoserine sites modify type IV pilus surface chemistry and fibre morphology. Mol. Microbiol. 31, 743–752 (1999). The covalently linked phosphate at Ser63 of GC pilin was removed by mutation (Ser to Ala), producing pili that were functionally indistinguishable from the wild type in vitro , but were less straight and more bundled, indicating that the phosphate might have a minor role in pilus assembly.
Zhang, H. Z. & Donnenberg, M. S. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol. Microbiol. 21, 787–797 (1996).
Kirn, T. J., Lafferty, M. J., Sandoe, C. M. & Taylor, R. K. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35, 896–910 (2000). Discrete functional domains are identified in the D-region of V. cholerae TcpA. The first two-thirds of the D-region are required for pilin folding and/or pilus assembly and are predicted to be buried in the TCP filament, and the last third of the D-region is involved in pilus–pilus interactions and predicted to be surface-exposed.
Richardson, J. S. The anatomy and taxonomy of protein structure. Adv. Protein Chem. 34, 167–339 (1981).
Lee, K. K. et al. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11, 705–713 (1994). The authors present strong evidence that the receptor-binding region of PAK pilin is buried along the length of the pilus filament and only exposed at its tip.
Krivan, H. C., Roberts, D. D. & Ginsburg, V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc β-1–4Gal found in some glycolipids. Proc. Natl Acad. Sci. USA 85, 6157–6161 (1988).
Ramphal, R., Sadoff, J. C., Pyle, M. & Silipigni, J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect. Immun. 44, 38–40 (1984).
Sun, D., Seyer, J. M., Kovari, I., Siumrada, R. A. & Taylor, R. K. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect. Immun. 59, 114–118 (1991).
Virji, M. & Heckels, J. E. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J. Gen. Microbiol. 130, 1089–1095 (1984).
Forest, K. T. et al. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect. Immun. 64, 644–652 (1996).
Watts, T. H., Kay, C. M. & Paranchych, W. Dissociation and characterization of pilin isolated from Pseudomonas aeruginosa strains PAK and PAO. Can. J. Biochem. 60, 867–872 (1982).
Watts, T. H., Kay, C. M. & Paranchych, W. Spectral properties of three quaternary arrangements of Pseudomonas pilin. Biochemistry 22, 3640–3646 (1983).
Bayley, D. P. & Jarrell, K. F. Further evidence to suggest that archaeal flagella are related to bacterial type IV pili. J. Mol. Evol. 46, 370–373 (1998).
Cohen-Krausz, S. & Trachtenberg, S. The structure of the archeabacterial flagellar filament of the extreme halophile Halobacterium salinarum R1M1 and its relation to eubacterial flagellar filaments and type IV pili. J. Mol. Biol. 321, 383–395 (2002).
Bardy, S. L., Ng, S. Y. & Jarrell, K. F. Prokaryotic motility structures. Microbiology 149, 295–304 (2003).
Correia, J. D. & Jarrell, K. F. Posttranslational processing of Methanococcus voltae preflagellin by preflagellin peptidases of M. voltae and other methanogens. J. Bacteriol. 182, 855–858 (2000).
Peabody, C. R. et al. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 149, 3051–3072 (2003).
Nunn, D. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol. 9, 402–408 (1999).
Sandkvist, M. Biology of type II secretion. Mol. Microbiol. 40, 271–283 (2001).
Sandkvist, M. Type II secretion and pathogenesis. Infect. Immun. 69, 3523–3535 (2001).
Sauvonnet, N., Vignon, G., Pugsley, A. P. & Gounon, P. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 19, 2221–2228 (2000). First demonstration that pseudopilins can form pilus-like 'pseudo-pili', achieved by overexpressing the 15 genes encoding the Klebsiella oxytoca pullulanase type II secreton in E. coli . This secreton also assembled the type IV pilin PpdD into pilus filaments.
Vignon, G. et al. Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides. J. Bacteriol. 185, 3416–3428 (2003).
Durand, E. et al. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J. Bacteriol. 185, 2749–2758 (2003).
Tsui, I. S., Yip, C. M., Hackett, J. & Morris, C. The type IVB pili of Salmonella enterica serovar Typhi bind to the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 71, 6049–6050 (2003).
Tobe, T. & Sasakawa, C. Role of bundle-forming pilus of enteropathogenic Escherichia coli in host cell adherence and in microcolony development. Cell. Microbiol. 3, 579–585 (2001).
Tobe, T. & Sasakawa, C. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell. Microbiol. 4, 29–42 (2002).
Doig, P. et al. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect. Immun. 56, 1641–1646 (1988).
Krivan, H. C., Ginsburg, V. & Roberts, D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch. Biochem. Biophys. 260, 493–496 (1988).
Ramphal, R. et al. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Gal β-1–3GlcNAc) or type 2 (Gal β-1–4GlcNAc) disaccharide units. Infect. Immun. 59, 700–704 (1991).
Doig, P., Paranchych, W., Sastry, P. A. & Irvin, R. T. Human buccal epithelial cell receptors of Pseudomonas aeruginosa: identification of glycoproteins with pilus binding activity. Can. J. Microbiol. 35, 1141–1145 (1989).
Lee, K. K., Doig, P., Irvin, R. T., Paranchych, W. & Hodges, R. S. Mapping the surface regions of Pseudomonas aeruginosa PAK pilin: the importance of the C-terminal region for adherence to human buccal epithelial cells. Mol. Microbiol. 3, 1493–1499 (1989).
Doig, P. et al. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect. Immun. 58, 124–130 (1990).
Farinha, M. A. et al. Alteration of the pilin adhesin of Pseudomonas aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect. Immun. 62, 4118–4123 (1994).
Wong, W. Y. et al. Structure–function analysis of the adherence-binding domain on the pilin of Pseudomonas aeruginosa strains PAK and KB7. Biochemistry 34, 12963–12972 (1995).
Sheth, H. B. et al. Development of an anti-adhesive vaccine for Pseudomonas aeruginosa targeting the C-terminal region of the pilin structural protein. Biomed. Pept. Proteins Nucleic Acids 1, 141–148 (1995).
Lepper, A. W., Moore, L. J., Atwell, J. L. & Tennent, J. M. The protective efficacy of pili from different strains of Moraxella bovis within the same serogroup against infectious bovine keratoconjunctivitis. Vet. Microbiol. 32, 177–187 (1992).
Hunt, J. D., Jackson, D. C., Brown, L. E., Wood, P. R. & Stewart, D. J. Antigenic competition in a multivalent foot rot vaccine. Vaccine 12, 457–464 (1994).
Egerton, J. R. et al. Protection of sheep against footrot with a recombinant DNA-based fimbrial vaccine. Vet. Microbiol. 14, 393–409 (1987).
Swanson, J., Robbins, K., Barrera, O. & Koomey, J. M. Gene conversion variations generate structurally distinct pilin polypeptides in Neisseria gonorrhoeae. J. Exp. Med. 165, 1016–1025 (1987).
Kellogg, D. S. Jr, Cohen, I. R., Norins, L. C., Schroeter, A. L. & Reising, G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J. Bacteriol. 96, 596–605 (1968).
Kallstrom, H., Liszewski, M. K., Atkinson, J. P. & Jonsson, A. B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25, 639–647 (1997). Identified the cell surface receptor for N. gonorrhoeae and established a direct interaction between GC pili and CD46.
Liszewski, M. K., Post, T. W. & Atkinson, J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9, 431–455 (1991).
Haas, R., Schwarz, H. & Meyer, T. F. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc. Natl Acad. Sci. USA 84, 9079–9083 (1987).
Rytkonen, A., Johansson, L., Asp, V., Albiger, B. & Jonsson, A. B. Soluble pilin of Neisseria gonorrhoeae interacts with human target cells and tissue. Infect. Immun. 69, 6419–6426 (2001).
Rudel, T., van Putten, J. P., Gibbs, C. P., Haas, R. & Meyer, T. F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol. Microbiol. 6, 3439–3450 (1992).
Scheuerpflug, I., Rudel, T., Ryll, R., Pandit, J. & Meyer, T. F. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect. Immun. 67, 834–843 (1999).
Nassif, X. et al. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc. Natl Acad. Sci. USA 91, 3769–3773 (1994).
Rudel, T., Scheurerpflug, I. & Meyer, T. F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 373, 357–359 (1995). Showed that PilC is present at the ends of N. gonorrhoea type IV pili, and implicated this protein in adhesion by showing PilC-mediated inhibition of pilus-mediated attachment of N. gonorrhoeae and N. meningitidis to human epithelial cells.
Jonsson, A. B., Ilver, D., Falk, P., Pepose, J. & Normark, S. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol. Microbiol. 13, 403–416 (1994).
Jonsson, A. B., Nyberg, G. & Normark, S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 10, 477–488 (1991).
Winther-Larsen, H. C. et al. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl Acad. Sci. USA 98, 15276–15281 (2001).
Hagblom, P., Segal, E., Billyard, E. & So, M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature 315, 156–158 (1985). Identified constant and antigenically variable regions of N. gonorrhoeae type IV pilin and showed that during the course of a human gonococcal infection, N. gonorrhoeae variants give rise to new isolates expressing altered pili.
Segal, E., Billyard, E., So, M., Storzbach, S. & Meyer, T. F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40, 293–300 (1985).
Long, C. D., Madraswala, R. N. & Seifert, H. S. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect. Immun. 66, 1918–1927 (1998).
Stern, A., Nickel, P., Meyer, T. F. & So, M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell 37, 447–456 (1984).
Boslego, J. W. et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9, 154–162 (1991).
Rytkonen, A. et al. Neisseria meningitidis undergoes PilC phase variation and PilE sequence variation during invasive disease. J. Infect. Dis. 189, 402–409 (2004).
Comer, J. E., Marshall, M. A., Blanch, V. J., Deal, C. D. & Castric, P. Identification of the Pseudomonas aeruginosa 1244 pilin glycosylation site. Infect. Immun. 70, 2837–2845 (2002).
Castric, P., Cassels, F. J. & Carlson, R. W. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J. Biol. Chem. 276, 26479–26485 (2001).
Kaiser, D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 76, 5952–5956 (1979).
Sun, H., Zusman, D. R. & Shi, W. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 10, 1143–1146 (2000).
Skerker, J. M. & Berg, H. C. Direct observation of extension and retraction of type IV pili. Proc. Natl Acad. Sci. USA 98, 6901–6904 (2001). A direct demonstration that twitching motility is driven by type IV pilus retraction. Total internal reflection microscopy was used to record extension and retraction of fluorescently labelled type IV pili at rates of 0.5 μm s−1 in P. aeruginosa , and showed that pilus retraction propelled cells forward.
Whitchurch, C. B., Hobbs, M., Livingston, S. P., Krishnapillai, V. & Mattick, J. S. Characterisation of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system widespread in eubacteria. Gene 101, 33–44 (1991). Identified the gene for PilT and established a genetic relationship between the type IV pili and the type II secretion system on the basis of homology of assembly components.
Wolfgang, M., van Putten, J. P., Hayes, S. F., Dorward, D. & Koomey, M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19, 6408–6418 (2000).
Kaiser, D. Bacterial motility: how do pili pull? Curr. Biol. 10, R777–R780 (2000).
Fussenegger, M., Rudel, T., Barten, R., Ryll, R. & Meyer, T. F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae — a review. Gene 192, 125–134 (1997).
Vuopio-Varkila, J. & Schoolnik, G. K. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J. Exp. Med. 174, 1167–1177 (1991).
Cravioto, A., Gross, R. J., Scotland, S. M. & Rowe, B. An adhesive factor found in strains of Escherichia coli belonging to the tradiitional infantile enteropathogenic serotypes. Curr. Microbiol. 3, 95–99 (1979).
Scaletsky, I. C., Silva, M. L. & Trabulsi, L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45, 534–536 (1984).
Hicks, S., Frankel, G., Kaper, J. B., Dougan, G. & Phillips, A. D. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66, 1570–1578 (1998).
Donnenberg, M. S., Kaper, J. B. & Finlay, B. B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5, 109–114 (1997).
Nataro, J. P. et al. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr. Infect. Dis. J. 6, 829–831 (1987).
Knutton, S., Shaw, R. K., Anantha, R. P., Donnenberg, M. S. & Zorgani, A. A. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33, 499–509 (1999).
O'Toole, G. A. & Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30, 295–304 (1998).
Klausen, M., Aaes-Jorgensen, A., Molin, S. & Tolker-Nielsen, T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50, 61–68 (2003).
Freitag, N. E., Seifert, H. S. & Koomey, M. Characterization of the pilF–pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 16, 575–586 (1995).
Tonjum, T., Freitag, N. E., Namork, E. & Koomey, M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol. Microbiol. 16, 451–464 (1995).
Drake, S. L. & Koomey, M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18, 975–986 (1995).
Aas, F. E. et al. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol. Microbiol. 46, 749–760 (2002). Demonstrated that DNA binding and uptake occur in two discrete steps, with DNA binding requiring type IV pili and ComP, and DNA uptake requiring functional PilT.
Karaolis, D. K., Somara, S., Maneval, D. R. Jr, Johnson, J. A. & Kaper, J. B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature 399, 375–379 (1999).
Waldor, M. K. & Mekalanos, J. J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914 (1996). Identified the filamentous phage CTXφ, which carries the structural genes for cholera toxin, and showed that CTXφ uses the V. cholerae TCP as its receptor.
Bradley, D. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology 51, 489–492 (1973).
Sun, T. P. & Webster, R. E. Nucleotide sequence of a gene cluster involved in entry of colicins and single–stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J. Bacteriol. 169, 2667–2674 (1987).
Novotny, C. P. & Fives-Taylor, P. Retraction of F pili. J. Bacteriol. 117, 1306–1311 (1974).
Jacobson, A. Role of F pili in the penetration of bacteriophage f1. J. Virol. 10, 835–843 (1972).
Ayala, B. P. et al. The pilus-induced Ca2+ flux triggers lysosome exocytosis and increases the amount of Lamp1 accessible to Neisseria IgA1 protease. Cell. Microbiol. 3, 265–275 (2001).
Kallstrom, H. & Jonsson, A. B. Characterization of the region downstream of the pilus biogenesis gene pilC1 in Neisseria gonorrhoeae. Biochim. Biophys. Acta 1397, 137–140 (1998).
Jendrossek, V. et al. Apoptotic response of Chang cells to infection with Pseudomonas aeruginosa strains PAK and PAO-I: molecular ordering of the apoptosis signaling cascade and role of type IV pili. Infect. Immun. 71, 2665–2673 (2003).
Abul-Milh, M., Wu, Y., Lau, B., Lingwood, C. A. & Foster, D. B. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili. Infect. Immun. 69, 7356–7364 (2001).
Gill, D. B., Koomey, M., Cannon, J. G. & Atkinson, J. P. Down-regulation of CD46 by piliated Neisseria gonorrhoeae. J. Exp. Med. 198, 1313–1322 (2003).
Blom, A. M. et al. A novel interaction between type IV pili of Neisseria gonorrhoeae and the human complement regulator C4B-binding protein. J. Immunol. 166, 6764–6770 (2001).
Acknowledgements
We thank K. Forest and M. Donnenberg for valuable discussions, A. Yamagata, D. Shin and J. Huffman for comments, and the National Institute of Allergy and Infectious Diseases and the Canadian Institutes of Health Research for funding our research on type IV pilus structure and assembly.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Related links
DATABASES
Entrez
Salmonella enterica serovar Typhi
The Protein Data Bank
FURTHER INFORMATION
Glossary
- AMPHIPATHIC
-
Having both hydrophobic and hydrophilic regions.
- SCAFFOLD
-
A supporting structure.
- SYMMETRY OPERATORS
-
A set of operations or moves, such as translations and rotations, about a symmetry axis, which convert one object into another apparently identical object.
- AXIAL TRANSLATION
-
Also known as rise, it is the axial distance between one subunit and the next in a given start.
- AZIMUTHAL ROTATION
-
Also known as twist, it is a rotation about the helical axis from one subunit to the next in a given start.
- EQUATORIAL
-
A horizontal line through the centre of the diffraction pattern. can be compared with the meridian, which is a vertical line through the centre of the diffraction pattern.
- MERIDIONAL INTENSITY
-
An intensity or layer line that coincides with the meridian of a diffraction pattern.
- PITCH
-
The axial distance for one helical turn.
- LAYER LINE
-
A pair of lines, arranged symmetrically about the origin of a diffraction pattern or Fourier transform that correspond to a helical wave. Layer lines are perpendicular to the direction of the helical axis and are spaced axially at multiples of the reciprocal of the helical pitch.
- ONE-START
-
A one-start helix, also known as the 'genetic' or 'primitive' helix, is the helical path that connects every subunit with the smallest axial rotation.
- THREE-START
-
A three-start helix is a set of three identical helices, all having the same pitch and number of subunits per turn, which together connect all subunits in the filament.
Rights and permissions
About this article
Cite this article
Craig, L., Pique, M. & Tainer, J. Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol 2, 363–378 (2004). https://doi.org/10.1038/nrmicro885
Issue Date:
DOI: https://doi.org/10.1038/nrmicro885
This article is cited by
-
Bacterial motility: machinery and mechanisms
Nature Reviews Microbiology (2022)
-
Characterization of Stenotrophomonas maltophilia phage AXL1 as a member of the genus Pamexvirus encoding resistance to trimethoprim–sulfamethoxazole
Scientific Reports (2022)
-
Phylogenetic analysis and characterization of arsenic (As) transforming bacterial marker proteins following isolation of As-tolerant indigenous bacteria
Archives of Microbiology (2022)
-
The effect of Quorum sensing inhibitors on the evolution of CRISPR-based phage immunity in Pseudomonas aeruginosa
The ISME Journal (2021)
-
Structure of Geobacter pili reveals secretory rather than nanowire behaviour
Nature (2021)