Key Points
-
Apolipoprotein E4 (APOE4) is the strongest risk factor for sporadic late-onset Alzheimer's disease (AD), which accounts for the vast majority of AD cases.
-
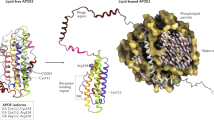
APOE4 differs from APOE2 and APOE3 at amino acid positions 112 and 158 and has a unique conformation that influences its lipid- and receptor-binding properties.
-
The cellular functions of APOE are mediated by APOE receptors, which are members of the low-density lipoprotein receptor (LDLR) family. LDLR-related protein 1 (LRP1) and the LDLRs are the two major types of APOE metabolic receptors in the brain.
-
APOE receptors regulate amyloid precursor protein (APP) trafficking and processing to amyloid-β (Aβ). Some of these functions are further modified by particular APOE isoforms.
-
APOE and APOE receptors have important roles in Aβ clearance both in the brain parenchyma and in the brain vasculature. APOE3 binds to Aβ more strongly than APOE4, and therefore it is more efficient at mediating Aβ clearance through APOE receptors.
-
APOE fragments generated from APOE4 influence tau phosphorylation and mitochondrial function. However, the mechanisms of these events are poorly understood.
-
The primary function of APOE is to transport lipids from astrocytes to neurons, an event that is crucial for synaptogenesis, synaptic repair, dendritic spine integrity and synaptic functions. APOE4 functions less efficiently than APOE3 in these processes.
-
APOE and APOE receptors are new targets for AD therapy. Several strategies have been reported or proposed.
Abstract
The vast majority of Alzheimer's disease (AD) cases are late-onset and their development is probably influenced by both genetic and environmental risk factors. A strong genetic risk factor for late-onset AD is the presence of the ɛ4 allele of the apolipoprotein E (APOE) gene, which encodes a protein with crucial roles in cholesterol metabolism. There is mounting evidence that APOE4 contributes to AD pathogenesis by modulating the metabolism and aggregation of amyloid-β peptide and by directly regulating brain lipid metabolism and synaptic functions through APOE receptors. Emerging knowledge of the contribution of APOE to the pathophysiology of AD presents new opportunities for AD therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Selkoe, D. J. Deciphering the genesis and fate of amyloid β-protein yields novel therapies for Alzheimer disease. J. Clin. Invest. 110, 1375–1381 (2002).
Blennow, K., de Leon, M. J. & Zetterberg, H. Alzheimer's disease. Lancet 368, 387–403 (2006).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Selkoe, D. & Kopan, R. Notch and presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 26, 565–597 (2003).
Shen, J. & Kelleher, R. J. The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc. Natl Acad. Sci. USA 104, 403–409 (2007).
Strittmatter, W. J. et al. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 1977–1981 (1993). References 6 and 7 were the first to report a genetic association between the ɛ4 allele of the APOE gene and LOAD.
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993).
Mahley, R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 (1988). A classic review on the biochemical properties and cholesterol transport function of APOE.
Bertram, L. & Tanzi, R. E. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nature Rev. Neurosci. 9, 768–778 (2008). This paper provides a comprehensive review of the current state of AD genetics.
Blacker, D. et al. ApoE-4 and age at onset of Alzheimer's disease: the NIMH genetics initiative. Neurology 48, 139–147 (1997).
Burt, T. D. et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE ɛ4/ɛ4 genotype accelerates HIV disease progression. Proc. Natl Acad. Sci. USA 105, 8718–8723 (2008).
Greenberg, S. M., Rebeck, G. W., Vonsattel, J. P., Gomez-Isla, T. & Hyman, B. T. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann. Neurol. 38, 254–259 (1995).
Josephs, K. A., Tsuboi, Y., Cookson, N., Watt, H. & Dickson, D. W. Apolipoprotein E ɛ4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch. Neurol. 61, 1579–1584 (2004).
Martinez, M. et al. Apolipoprotein E4 is probably responsible for the chromosome 19 linkage peak for Parkinson's disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 136B, 172–174 (2005).
Masterman, T. & Hillert, J. The telltale scan: APOE ɛ4 in multiple sclerosis. Lancet Neurol. 3, 331 (2004).
Herz, J. & Bock, H. H. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 71, 405–434 (2002).
Herz, J. & Chen, Y. Reelin, lipoprotein receptors and synaptic plasticity. Nature Rev. Neurosci. 7, 850–859 (2006).
Pitas, R. E., Boyles, J. K., Lee, S. H., Foss, D. & Mahley, R. W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta 917, 148–161 (1987).
Uchihara, T. et al. ApoE immunoreactivity and microglial cells in Alzheimer's disease brain. Neurosci. Lett. 195, 5–8 (1995).
Pitas, R. E., Boyles, J. K., Lee, S. H., Hui, D. & Weisgraber, K. H. Lipoproteins and their receptors in the central nervous system. J. Biol. Chem. 262, 14352–14360 (1987).
LaDu, M. J. et al. Nascent astrocyte particles differ from lipoproteins in CSF. J. Neurochem. 70, 2070–2081 (1998).
Hirsch-Reinshagen, V. et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279, 41197–41207 (2004).
Wahrle, S. E. et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279, 40987–40993 (2004).
Hatters, D. M., Peters-Libeu, C. A. & Weisgraber, K. H. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 31, 445–454 (2006). A comprehensive review of the biophysical and structural properties of the APOE isoforms.
Zhong, N. & Weisgraber, K. H. Understanding the association of apolipoprotein E4 with Alzheimer's disease: clues from its structure. J. Biol. Chem. 284, 6027–6031 (2009).
Weisgraber, K. H., Rall, S. C. Jr & Mahley, R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J. Biol. Chem. 256, 9077–9083 (1981).
Ramaswamy, G., Xu, Q., Huang, Y. & Weisgraber, K. H. Effect of domain interaction on apolipoprotein E levels in mouse brain. J. Neurosci. 25, 10658–10663 (2005).
Goldstein, J. L., Brown, M. S., Anderson, R. G. W., Russell, D. W. & Schneider, W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu. Rev. Cell Biol. 1, 1–39 (1985).
Jeon, H. & Blacklow, S. C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 74, 535–562 (2005).
Herz, J. et al. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7, 4119–4127 (1988). This paper reported the initial cloning and structural properties of LRP1, a major APOE receptor in the brain.
Herz, J., Kowal, R. C., Goldstein, J. L. & Brown, M. S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 9, 1769–1776 (1990).
Li, Y., Lu, W., Marzolo, M. P. & Bu, G. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J. Biol. Chem. 276, 18000–18006 (2001).
Herz, J., Clouthier, D. E. & Hammer, R. E. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71, 411–421 (1992).
May, P. et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 24, 8872–8883 (2004).
Willnow, T. E., Nykjaer, A. & Herz, J. Lipoprotein receptors: new roles for ancient proteins. Nature Cell Biol. 1, E157–E162 (1999).
Liu, C. X., Li, Y., Obermoeller-McCormick, L. M., Schwartz, A. L. & Bu, G. The putative tumor suppressor LRP1B, a novel member of the low density lipoprotein (LDL) receptor family, exhibits both overlapping and distinct properties with the LDL receptor-related protein. J. Biol. Chem. 276, 28889–28896 (2001).
Li, Y. et al. Low density lipoprotein (LDL) receptor-related protein 1B impairs urokinase receptor regeneration on the cell surface and inhibits cell migration. J. Biol. Chem. 277, 42366–42371 (2002).
Cam, J. A. et al. The low density lipoprotein receptor-related protein 1B retains β-amyloid precursor protein at the cell surface and reduces amyloid-β peptide production. J. Biol. Chem. 279, 29639–29646 (2004).
Trommsdorff, M. et al. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 (1999). This paper described the role of APOER2 and VLDLR function in reelin signalling, which is crucial for neuronal migration during development.
Johnson, E. B., Hammer, R. E. & Herz, J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum. Mol. Genet. 14, 3523–3538 (2005).
Kim, N. et al. Lrp4 is a receptor for agrin and forms a complex with MuSK. Cell 135, 334–342 (2008).
He, X., Semenov, M., Tamai, K. & Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131, 1663–1677 (2004).
May, P. & Herz, J. LDL receptor-related proteins in neurodevelopment. Traffic 4, 291–301 (2003).
Bu, G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr. Opin. Lipidol. 9, 149–155 (1998).
Bu, G. & Marzolo, M. P. Role of rap in the biogenesis of lipoprotein receptors. Trends Cardiovasc. Med. 10, 148–155 (2000).
Fryer, J. D. et al. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J. Biol. Chem. 280, 25754–25759 (2005).
Liu, Q. et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 56, 66–78 (2007). This paper reported that APP and APOE are functionally linked in brain cholesterol metabolism through the APOE receptor LRP1.
Zerbinatti, C. V. et al. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Aβ42 accumulation in amyloid model mice. J. Biol. Chem. 281, 36180–36186 (2006).
Rebeck, G. W., Reiter, J. S., Strickland, D. K. & Hyman, B. T. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron 11, 575–580 (1993). This paper described the finding that both APOE and its receptor LRP1 are present in amyloid plaques.
Rapp, A., Gmeiner, B. & Huttinger, M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 88, 473–483 (2006).
Narita, M. et al. Cellular catabolism of lipid poor apolipoprotein E via cell surface LDL receptor-related protein. J. Biochem. 132, 743–749 (2002).
Kowal, R. C. et al. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 265, 10771–10779 (1990).
Fagan, A. M., Bu, G. J., Sun, Y. L., Daugherty, A. & Holtzman, D. M. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J. Biol. Chem. 271, 30121–30125 (1996).
Niu, S., Yabut, O. & D'Arcangelo, G. The reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 28, 10339–10348 (2008).
Beffert, U. et al. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J. Neurosci. 24, 1897–1906 (2004).
Beffert, U. et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47, 567–579 (2005).
D'Arcangelo, G. et al. Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 (1999).
Hoe, H. S., Harris, D. C. & Rebeck, G. W. Multiple pathways of apolipoprotein E signaling in primary neurons. J. Neurochem. 93, 145–155 (2005).
Qiu, Z., Crutcher, K. A., Hyman, B. T. & Rebeck, G. W. ApoE isoforms affect neuronal N-methyl-D-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience 122, 291–303 (2003).
Hayashi, H., Campenot, R. B., Vance, D. E. & Vance, J. E. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 27, 1933–1941 (2007).
Kounnas, M. Z. et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 82, 331–340 (1995). The first report to describe an interaction between APP and LRP1. Subsequent studies showed that LRP1 regulates APP trafficking and processing.
Trommsdorff, R., Borg, J. P., Margolis, B. & Herz, J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 273, 33556–33560 (1998).
Pietrzik, C. U. et al. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 24, 4259–4265 (2004).
Kinoshita, A. et al. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci. 21, 8354–8361 (2001).
Ulery, P. G. et al. Modulation of β-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J. Biol. Chem. 275, 7410–7415 (2000).
Cam, J. A., Zerbinatti, C. V., Li, Y. & Bu, G. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the β-amyloid precursor protein. J. Biol. Chem. 280, 15464–15470 (2005).
Cole, S. L. & Vassar, R. The Alzheimer's disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2, 22 (2007).
Zerbinatti, C. V. et al. Increased soluble amyloid-β peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc. Natl Acad. Sci. USA 101, 1075–1080 (2004).
Ye, S. et al. Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proc. Natl Acad. Sci. USA 102, 18700–18705 (2005).
Hoe, H. S. et al. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 25, 9259–9268 (2005).
Scherzer, C. R. et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch. Neurol. 61, 1200–1205 (2004).
Andersen, O. M. et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl Acad. Sci. USA 102, 13461–13466 (2005).
Rogaeva, E. et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nature Genet. 39, 168–177 (2007).
Cirrito, J. R. et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 23, 8844–8853 (2003). This paper described an in vivo microdialysis technique to assess Aβ concentration in brain interstitial fluid. It also showed that Aβ's half-life in the mouse brain is 2–4 h.
Bateman, R. J. et al. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nature Med. 12, 856–861 (2006).
Deane, R. et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43, 333–344 (2004). This paper reported an important function of LRP1 in brain Aβ efflux through the BBB. It also demonstrated that Aβ binds directly to LRP1.
Strittmatter, W. J. et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer's disease. Proc. Natl Acad. Sci. USA 90, 8098–8102 (1993).
Tamamizu-Kato, S. et al. Interaction with amyloid β peptide compromises the lipid binding function of apolipoprotein E. Biochemistry 47, 5225–5234 (2008).
Beffert, U. et al. β-amyloid peptides increase the binding and internalization of apolipoprotein E to hippocampal neurons. J. Neurochem. 70, 1458–1466 (1998).
Hone, E. et al. Alzheimer's disease amyloid-β peptide modulates apolipoprotein E isoform specific receptor binding. J. Alzheimers Dis. 7, 303–314 (2005).
LaDu, M. J. et al. Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 269, 23403–23406 (1994). This paper showed that APOE3–lipoprotein binds to Aβ with higher affinity than APOE4–lipoprotein.
Holtzman, D. M. et al. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer's disease. J. Clin. Invest. 103, R15–R21 (1999).
Holtzman, D. M. et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 97, 2892–2897 (2000). This paper described the differential in vivo effects of human APOE isoforms on amyloid deposition in mouse models.
DeMattos, R. B. et al. ApoE and clusterin cooperatively suppress Aβ levels and deposition: evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron 41, 193–202 (2004).
Schmechel, D. E. et al. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 9649–9653 (1993).
Bogdanovic, N., Corder, E., Lannfelt, L. & Winblad, B. APOE polymorphism and clinical duration determine regional neuropathology in Swedish APP(670, 671) mutation carriers: implications for late-onset Alzheimer's disease. J. Cell. Mol. Med. 6, 199–214 (2002).
Small, G. W. et al. Influence of cognitive status, age, and APOE-4 genetic risk on brain FDDNP positron-emission tomography imaging in persons without dementia. Arch. Gen. Psychiatry 66, 81–87 (2009).
Deane, R. et al. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 118, 4002–4013 (2008).
Van Uden, E. et al. Increased extracellular amyloid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein. J. Neurosci. 22, 9298–9304 (2002).
Shibata, M. et al. Clearance of Alzheimer's amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499 (2000).
Billings, L. M., Oddo, S., Green, K. N., McGaugh, J. L. & Laferla, F. M. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45, 675–688 (2005).
Koistinaho, M. et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nature Med. 10, 719–726 (2004). This paper showed that APOE that is secreted by astrocytes has a crucial role in degrading Aβ that is associated with amyloid plaques; this process depends on the function of APOE receptors.
Khoury, J. E. & Luster, A. D. Mechanisms of microglia accumulation in Alzheimer's disease: therapeutic implications. Trends Pharmacol. Sci. 29, 626–632 (2008).
McCarron, M. O. & Nicoll, J. A. R. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 3, 484–492 (2004).
Roher, A. E. et al. β-amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 10836–10840 (1993).
Fryer, J. D. et al. Human apolipoprotein E4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J. Neurosci. 25, 2803–2810 (2005).
Leissring, M. A. The ABCs of Aβ-cleaving proteases. J. Biol. Chem. 283, 29645–29649 (2008).
Tanzi, R. E., Moir, R. D. & Wagner, S. L. Clearance of Alzheimer's Aβ peptide: the many roads to perdition. Neuron 43, 605–608 (2004).
Jiang, Q. et al. ApoE promotes the proteolytic degradation of Aβ. Neuron 58, 681–693 (2008).
Dahlgren, K. N. et al. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 277, 32046–32053 (2002).
Manelli, A. M., Bulfinch, L. C., Sullivan, P. M. & LaDu, M. J. Aβ42 neurotoxicity in primary co-cultures: effect of apoE isoform and Aβ conformation. Neurobiol. Aging 28, 1139–1147 (2007).
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Med. 14, 837–842 (2008). References 102 and 103 demonstrated that Aβ oligomers isolated from the brain are highly neurotoxic.
Lesne, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006).
Belinson, H., Lev, D., Masliah, E. & Michaelson, D. M. Activation of the amyloid cascade in apolipoprotein E4 transgenic mice induces lysosomal activation and neurodegeneration resulting in marked cognitive deficits. J. Neurosci. 28, 4690–4701 (2008). This paper showed that APOE4 and Aβ synergistically activate neurotoxic pathways that lead to neurodegeneration and cognitive deficits in mice.
Muller, T., Meyer, H. E., Egensperger, R. & Marcus, K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer's disease. Prog. Neurobiol. 85, 393–406 (2008).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 (2007).
Tesseur, I. et al. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am. J. Pathol. 156, 951–964 (2000).
Brecht, W. J. et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 24, 2527–2534 (2004). This paper demonstrated that neuronal expression of APOE4 leads to increased tau phosphorylation.
Aoki, K. et al. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke 34, 875–880 (2003).
Xu, Q. et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J. Neurosci. 26, 4985–4994 (2006).
Chang, S. et al. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc. Natl Acad. Sci. USA 102, 18694–18699 (2005).
Mahley, R. W., Weisgraber, K. H. & Huang, Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl Acad. Sci. USA 103, 5644–5651 (2006).
Pfrieger, F. W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 60, 1158–1171 (2003).
Mauch, D. H. et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 (2001). This paper showed that astrocyte-secreted cholesterol is essential for synaptogenesis.
Shobab, L. A., Hsiung, G.-Y. R. & Feldman, H. H. Cholesterol in Alzheimer's disease. Lancet Neurol. 4, 841–852 (2005).
Puglielli, L., Tanzi, R. E. & Kovacs, D. M. Alzheimer's disease: the cholesterol connection. Nature Neurosci. 6, 345–351 (2003).
Kaether, C. & Haass, C. A lipid boundary separates APP and secretases and limits amyloid β-peptide generation. J. Cell Biol. 167, 809–812 (2004).
Reid, P. C., Urano, Y., Kodama, T. & Hamakubo, T. Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. J. Cell. Mol. Med. 11, 383–392 (2007).
Marzolo, M. P. & Bu, G. Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer's disease. Semin. Cell Dev. Biol. 17 Oct 2008 (doi: 10.1016/j.semcdb.2008.10.005).
Thinakaran, G. & Koo, E. H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283, 29615–29619 (2008).
Ledesma, M. D. et al. Raft disorganization leads to reduced plasmin activity in Alzheimer's disease brains. EMBO Rep. 4, 1190–1196 (2003).
Ledesma, M. D. & Dotti, C. G. Amyloid excess in Alzheimer's disease: what is cholesterol to be blamed for? FEBS Lett. 580, 5525–5532 (2006).
Hamanaka, H. et al. Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum. Mol. Genet. 9, 353–361 (2000).
Michikawa, M., Fan, Q. W., Isobe, I. & Yanagisawa, K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J. Neurochem. 74, 1008–1016 (2000).
Gong, J. S. et al. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J. Biol. Chem. 277, 29919–29926 (2002).
Han, X. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer's disease: a tale of shotgun lipidomics. J. Neurochem. 103, 171–179 (2007).
Selkoe, D. J. Alzheimer's disease is a synaptic failure. Science 298, 789–791 (2002).
Wang, C. et al. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol. Dis. 18, 390–398 (2005).
Trommer, B. L. et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport 15, 2655–2658 (2004).
Hayashi, T. et al. Different expression of low density lipoprotein receptor and ApoE between young adult and old rat brains after ischemia. Neurol. Res. 28, 822–825 (2006).
Holtzman, D. M. et al. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl Acad. Sci. USA 92, 9480–9484 (1995).
Nathan, B. P. et al. Differential effects of apolipoprotein E3 and E4 on neuronal growth in vitro. Science 264, 850–852 (1994). References 131 and 132 described the differential effects of APOE isoforms in promoting neurite outgrowth.
Ji, Y. et al. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer's disease patients. Neuroscience 122, 305–315 (2003).
Lanz, T. A., Carter, D. B. & Merchant, K. M. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 13, 246–253 (2003).
Brodbeck, J. et al. Rosiglitazone increases dendritic spine density and rescues spine loss caused by apolipoprotein E4 in primary cortical neurons. Proc. Natl Acad. Sci. USA 105, 1343–1346 (2008).
Laws, S. M., Hone, E., Gandy, S. & Martins, R. N. Expanding the association between the APOE gene and the risk of Alzheimer's disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J. Neurochem. 84, 1215–1236 (2003).
Riddell, D. R. et al. Impact of apolipoprotein E (apoE) polymorphism on brain apoE levels. J. Neurosci. 28, 11445–11453 (2008).
Vaya, J. & Schipper, H. M. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J. Neurochem. 102, 1727–1737 (2007).
Riddell, D. R. et al. The LXR agonist TO901317 selectively lowers hippocampal Aβ42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol. Cell. Neurosci. 34, 621–628 (2007).
Laskowitz, D. T. & Vitek, M. P. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics 8, 959–969 (2007).
Aono, M. et al. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience 116, 437–445 (2003).
Laskowitz, D. T., Fillit, H., Yeung, N., Toku, K. & Vitek, M. P. Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol. Scand. Suppl. 185, 15–20 (2006).
Bales, K. R. et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nature Genet. 17, 263–264 (1997).
Sadowski, M. J. et al. Blocking the apolipoprotein E/amyloid-β interaction as a potential therapeutic approach for Alzheimer's disease. Proc. Natl Acad. Sci. USA 103, 18787–18792 (2006).
Kang, D. E. et al. Modulation of amyloid β-protein clearance and Alzheimer's disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Invest. 106, 1159–1166 (2000).
Grimsley, P. G., Quinn, K. A. & Owensby, D. A. Soluble low-density lipoprotein receptor-related protein. Trends Cardiovasc. Med. 8, 363–368 (1998).
Quinn, K. A., Pye, V. J., Dai, Y. P., Chesterman, C. N. & Owensby, D. A. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP). Exp. Cell Res. 251, 433–441 (1999).
Sagare, A. et al. Clearance of amyloid-β by circulating lipoprotein receptors. Nature Med. 13, 1029–1031 (2007).
von Arnim, C. A. et al. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J. Biol. Chem. 280, 17777–17785 (2005).
May, P., Bock, H. H., Nimpf, J. & Herz, J. Differential glycosylation regulates processing of lipoprotein receptors by γ-secretase. J. Biol. Chem. 278, 37386–37392 (2003).
Kinoshita, A., Shah, T., Tangredi, M. M., Strickland, D. K. & Hyman, B. T. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J. Biol. Chem. 278, 41182–41188 (2003).
Zurhove, K., Nakajima, C., Herz, J., Bock, H. H. & May, P. γ-Secretase limits the inflammatory response through the processing of LRP1. Sci. Signal. 1, ra15 (2008).
Hoe, H. S. & Rebeck, G. W. Regulated proteolysis of APP and ApoE receptors. Mol. Neurobiol. 37, 64–72 (2008).
Acknowledgements
The author wishes to thank P. Tarr, D. Owyoung and members of the Bu laboratory for critical reading and comments on this Review. He apologizes to those whose work is not cited owing to either space limitations or the specific focus of the Review. Work in the author's laboratory is supported by the US National Institutes of Health (grants R01AG027924 and R01AG031784) and a Zenith Fellows Award from the Alzheimer's Association.
Author information
Authors and Affiliations
Supplementary information
Supplementary information S1 (table)
Ligands and functions of LDLR family members (PDF 170 kb)
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Polymorphic allele
-
A natural variant in a gene that occurs with fairly high frequency (> 1%) in the general population.
- Cerebral amyloid angiopathy
-
(CAA). A neurological condition in which amyloid protein is deposited onto the walls of the arteries of the brain. CAA increases the risk of bleeding into the brain, which causes haemorrhagic stroke.
- Molten globule
-
A partially folded protein state that is found in mildly denaturing conditions, such as low pH, the presence of a mild denaturant or high temperature. It is also used to refer to certain protein folding intermediates.
- PDAPP amyloid mouse model
-
A mouse model of AD in which a human APP transgene bearing the amyloidogenic V717F mutation is overexpressed under the control of the human platelet-derived growth factor-β chain gene promoter.
- Endopeptidase
-
An enzyme that catalyses the hydrolysis of peptide bonds in the interior of a polypeptide chain or protein molecule.
- PDGF-APPSw,Ind amyloid mouse model
-
A mouse model of AD in which a human APP transgene bearing the amyloidogenic V717F, K670N and M671L mutations is overexpressed under the control of the human platelet-derived growth factor-β chain gene promoter.
- Tg2576 amyloid mouse model
-
A mouse model of AD in which a human APP transgene bearing the amyloidogenic K670N and M671L mutations is overexpressed under the control of the hamster prion protein gene promoter.
- Protein prenylation and isoprenylation
-
The covalent attachment of hydrophobic prenyl or isoprenyl groups to a protein. These processes have roles in protein attachment to cell membranes and protein–protein interactions.
- ApoE4 targeted replacement (TR) mouse
-
A model of human APOE4 expression in which the human APOEε4 gene is inserted into the mouse Apoe gene locus. The expression of human APOE4 in these mice temporally and spatially resembles that of endogenous mouse APOE.
Rights and permissions
About this article
Cite this article
Bu, G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10, 333–344 (2009). https://doi.org/10.1038/nrn2620
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2620
This article is cited by
-
Biomarkers of UVB radiation-related senescent fibroblasts
Scientific Reports (2024)
-
Plasma Apo-E mediated corticospinal tract abnormalities and suicidality in patients with major depressive disorder
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Associations of screen-based sedentary activities with all cause dementia, Alzheimer’s disease, vascular dementia: a longitudinal study based on 462,524 participants from the UK Biobank
BMC Public Health (2023)
-
Role of neuroinflammation in neurodegeneration development
Signal Transduction and Targeted Therapy (2023)
-
Integrated multi-omics analysis of Alzheimer’s disease shows molecular signatures associated with disease progression and potential therapeutic targets
Scientific Reports (2023)