Key Points
-
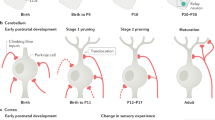
The transmitters expressed by neurons have been thought to be a constant and immutable aspect of neuronal identity, but recent work shows that electrical activity can respecify transmitter expression in both the developing and the mature nervous system.
-
This process seems to promote homeostatic equilibrium of excitability by changing the transmitters neurons synthesize and release, suggesting that this equilibrium is important for normal maturation of the nervous system and maintenance of homeostasis in circuits. Perturbations of embryonic activity with drugs may alter the composition and function of the nervous system by disturbing crucial homeostatic regulation of excitability.
-
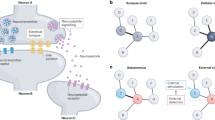
Transmitter respecification involves induction of transmitter expression in reserve pool neurons that already express other transmitters and are wired into circuits. Activity reprograms these neurons to express an additional transmitter or switch from one transmitter to another.
-
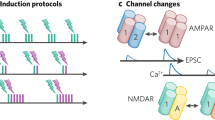
At the molecular level, activity engages the expression of transcription factors that alter the expression of genes encoding enzymes involved in neurotransmitter synthesis and transmitter transporters that drive transmitter respecification.
-
Activity-dependent changes in transmitter specification are matched by corresponding changes in the expression of the cognate postsynaptic receptors, enabling synaptic transmission by the novel transmitters.
-
Neurons expressing a new neurotransmitter can drive appropriate behaviours at early stages of development, putting activity-dependent transmitter specification on a functional map. Given that the structure and function of the nervous system track changes in the environment, it seems likely that activity-dependent changes in transmitter identity are a mechanism that enables behaviour to change flexibly.
-
Pathological loss of neurotransmitters and their receptors may be treated therapeutically by sensorimotor stimulation that drives transmitter respecification and hijacks existing neuronal circuitry.
Abstract
For many years it has been assumed that the identity of the transmitters expressed by neurons is stable and unchanging. Recent work, however, shows that electrical activity can respecify neurotransmitter expression during development and in the mature nervous system, and an understanding is emerging of the molecular mechanisms underlying activity-dependent transmitter respecification. Changes in postsynaptic neurotransmitter receptor expression accompany and match changes in transmitter specification, thus enabling synaptic transmission. The functional roles of neurotransmitter respecification are beginning to be understood and appear to involve homeostatic synaptic regulation, which in turn influences behaviour. Activation of this novel form of plasticity by sensorimotor stimuli may provide clinical benefits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Spitzer, N. C. & Lamborghini, J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc. Natl Acad. Sci. USA 73, 1641–1645 (1976).
Spitzer, N. C., Debaca, R. C., Allen, K. A. & Holliday, J. Calcium dependence of differentiation of GABA immunoreactivity in spinal neurons. J. Comp. Neurol. 337, 168–175 (1993).
Baccaglini, P. I. & Spitzer, N. C. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J. Physiol. 271, 93–117 (1977).
Roberts, A., Dale, N., Ottersen, O. P. & Storm-Mathisen, J. The early development of neurons with GABA immunoreactivity in the CNS of Xenopus laevis embryos. J. Comp. Neurol. 261, 435–449 (1987).
Dale, N., Roberts, A., Ottersen, O. P. & Storm-Mathisen, J. The development of a population of spinal cord neurons and their axonal projections revealed by GABA immunocytochemistry in frog embryos. Proc. R. Soc. Lond. B 232, 205–215 (1987).
Root, C. M. et al. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J. Neurosci. 28, 4777–4784 (2008). This study identified paracrine actions of GABA and glutamate as triggers for spontaneous calcium spikes that regulate neurotransmitter respecification.
Gu, X. & Spitzer, N. C. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature 375, 784–787 (1995). This paper provided the first demonstration of coding of neuronal differentiation by low-frequency, long-duration calcium transients.
Watt, S. D., Gu, X., Smith, R. D. & Spitzer N.C. Specific frequencies of spontaneous Ca2+ transients upregulate GAD 67 transcripts in embryonic spinal neurons. Mol. Cell. Neurosci. 16, 376–387 (2000).
Borodinsky, L. N. et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429, 523–530 (2004). This study demonstrated homeostatic neurotransmitter respecification in the developing vertebrate spinal cord in the absence of changes in molecular markers of cell identity.
Turrigiano, G. G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435 (2008).
Davis, G. W. Homeostatic control of neural activity: from phenomenology to molecular design. Ann. Rev. Neurosci. 29, 307–323 (2006).
Marder, E. Quantification of Behavior Sackler Colloquium: variability, compensation, and modulation in neurons and circuits. Proc. Natl Acad. Sci. USA 108 (Suppl. 3), 15542–15548 (2011).
Demarque, M. & Spitzer, N. C. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron 67, 321–334 (2010). This study identified a molecular mechanism by which activity regulates neurotransmitter respecification and revealed the behavioural impact of new neurotransmitter expression.
Dulcis, D. & Spitzer, N. C. Illumination controls dopaminergic differentiation regulating behavior. Nature 456, 195–201 (2008). This paper demonstrated that sensory input respecifies neurotransmitter expression and the expression of the cognate postsynaptic neurotransmitter receptors that enable camouflage behaviour.
Velázquez-Ulloa, N. A., Spitzer, N. C. & Dulcis, D. Contexts for dopamine specification by calcium spike activity in the CNS. J. Neurosci. 31, 78–88 (2011).
Baker, H. Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neuroscience 36, 761–771 (1990).
Dulcis, D. & Spitzer, N. C. Reserve pool neuron transmitter respecification: novel neuroplasticity. Dev. Neurobiol. 18 May 2011 (doi:10.1002/dneu.20920).
Ben-Ari, Y. & Spitzer, N. C. Phenotypic checkpoints regulate neuronal development. Trends Neurosci. 33, 485–492 (2010).
Hökfelt, T. et al. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc. Natl Acad. Sci. USA 74, 3587–3591 (1977).
Hökfelt, T. et al. Peptidergic neurones. Nature 284, 515–521 (1980).
Furshpan, E. J., MacLeish, P. R., O'Lague, P. H. & Potter, D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc. Natl Acad. Sci. USA 73, 4225–4229 (1976).
Potter, D. D., Landis, S. C. & Furshpan, E. J. Dual function during development of rat sympathetic neurones in culture. J. Exp. Biol. 89, 57–71 (1980).
Demarque, M. & Spitzer, N. C. Neurotransmitter phenotype plasticity: an unexpected mechanism in the toolbox of network activity homeostasis. Dev. Neurobiol. 72, 22–32 (2011).
Nishimaru, H. et al. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc. Natl Acad. Sci. USA 102, 5245–5249 (2005).
Mentis, G. Z. et al. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc. Natl Acad. Sci. USA 102, 7344–7349 (2005).
Soussi, R. et al. Heterogeneity of the supramammillary-hippocampal pathways: evidence for a unique GABAergic neurotransmitter phenotype and regional differences. Eur. J. Neurosci. 32, 771–785 (2010).
Gras, C. et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. 22, 5442–5451 (2002).
Amilhon, B. et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 30, 2198–2210 (2010).
Seal, R. P. & Edwards, R. H. Functional implications of neurotransmitter co-release: glutamate and GABA share the load. Curr. Opin. Pharmacol. 6, 114–119 (2006).
Chang, L. W. & Spitzer, N. C. Spontaneous calcium spike activity in embryonic spinal neurons is regulated by developmental expression of the Na+, K+-ATPase β3 subunit. J. Neurosci. 29, 7877–7885 (2009).
McCabe, A. K., Chisholm, S. L., Picken-Bahrey, H. L. & Moody, W. J. The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. J. Physiol. 577, 155–167 (2006).
Echelard, Y. et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993).
Roelink, H. et al. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 81, 445–455 (1995).
Ericson, J. et al. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell 87, 661–673 (1996).
Belgacem, Y. H. & Borodinsky, L. N. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc. Natl Acad. Sci. USA 108, 4482–4487 (2011). This study shows that the concentration of the morphogen SHH controls the frequency of calcium spikes that regulate neurotransmitter respecification.
De Koninck, P. & Schulman, H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230 (1998).
Oancea, E. & Meyer, T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell 95, 307–318 (1996).
Eshete, F. & Fields, R. D. Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. J. Neurosci. 21, 6694–6705 (2001).
Brosenitsch, T. A., Salgado-Commissariat, D., Kunze, D. L. & Katz, D. M. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. J. Neurosci. 18, 1047–1055 (1998).
Brosenitsch, T. A. & Katz, D. M. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J. Neurosci. 21, 2571–2579 (2001).
Chevalier, J. et al. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J. Physiol. 586, 1963–1975 (2008).
Belousov, A. B., O'Hara, B. F. & Denisova, J. V. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J. Neurosci. 21, 2015–2027 (2001).
Belousov, A. B., Hunt, N. D., Raju, R. P. & Denisova, J. V. Calcium-dependent regulation of cholinergic cell phenotype in the hypothalamus in vitro. J. Neurophysiol. 88, 1352–1362 (2002).
Liu, X. et al. Regulation of cholinergic phenotype in developing neurons. J. Neurophysiol. 99, 2443–2455 (2008). This paper demonstrated respecification of choline acetyltransferase expression in hypothalamic neurons following blockade or inactivation of NMDA receptors.
Thor, S. & Thomas, J. B. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron 18, 397–409 (1997).
Pierani, A. et al. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29, 367–384 (2001).
Tanabe, Y., William, C. & Jessell, T. M. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell 95, 67–80 (1998).
Roybon, L. et al. GABAergic differentiation induced by Mash1 is compromised by the bHLH proteins Neurogenin2, NeuroD1, and NeuroD2. Cereb. Cortex 20, 1234–1244 (2010).
Brosenitsch, T. A. & Katz, D. M. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Mol. Cell. Neurosci. 20, 447–457 (2002). This study showed that temporal and spatial expression of PHOX2 transcription factors is correlated with the potential for activity-dependent induction of the dopaminergic phenotype.
Cheng, L. et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nature Neurosci. 7, 510–517 (2004).
Cheng, L. et al. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nature Neurosci. 8, 1510–1515 (2005).
Marek, K. W., Kurtz, L. M. & Spitzer, N. C. cJun integrates calcium spike activity and tlx3 expression to regulate neurotransmitter specification. Nature Neurosci. 13, 944–950 (2010). This study identified a molecular mechanism for activity-dependent neurotransmitter respecification involving phosphorylation of c-Jun and its binding to a CRE site in the promoter of the glutamate/GABA selector gene tlx3.
Flames, N. & Hobert, O. Gene regulatory logic of dopamine neuron differentiation. Nature 458, 885–889 (2009).
Borodinsky, L. N. & Spitzer, N. C. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc. Natl Acad. Sci. USA 104, 335–340 (2007). This paper demonstrated the presence of non-cholinergic postsynaptic currents following activity-dependent neurotransmitter respecification in the embryonic spinal cord.
Marrus, S. B. & DiAntonio, A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr. Biol. 14, 924–931 (2004).
Kummer, T. T., Misgeld, T. & Sanes, J. R. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol. 16, 74–82 (2006).
Cesa, R., Morando, L. & Strata, P. Transmitter-receptor mismatch in GABAergic synapses in the absence of activity. Proc. Natl Acad. Sci. USA 105, 18988–18993 (2008).
Catalano, S. M., Chang, C. K. & Shatz, C. J. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J. Neurosci. 17, 8376–8390 (1997).
Gu, Q. et al. Effects of tetrodotoxin treatment in LGN on neuromodulatory receptor expression in developing visual cortex. Brain Res. Dev. Brain Res. 106, 93–99 (1998).
Shi, J., Townsend, M. & Constantine-Paton, M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron 28, 103–114 (2000).
Sillar, K. T., Reith, C. A. & McDearmid, J. R. Development and aminergic neuromodulation of a spinal locomotor network controlling swimming in Xenopus larvae. Ann. NY Acad. Sci. 860, 318–332 (1998).
Kosaka, T. et al. Differential effect of functional olfactory deprivation on the GABAergic and catecholaminergic traits in the rat main olfactory bulb. Brain Res. 413, 197–203 (1987).
Baker, H., Morel, K., Stone, D. M. & Maruniak, J. A. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 614, 109–116 (1993).
Tu, S., Butt, C. M., Pauly, J. R. & Debski, E. A. Activity-dependent regulation of substance P expression and topographic map maintenance by a cholinergic pathway. J. Neurosci. 20, 5346–5357 (2000).
Gutiérrez, R. Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J. Neurophysiol. 84, 3088–3090 (2000).
Gutiérrez, R. Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibers in vitro. J. Neurophysiol. 87, 2562–2570 (2002). This study demonstrated activity-dependent neurotransmitter switching in the adult rat brain.
Gutiérrez, R. et al. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J. Neurosci. 23, 5594–5598 (2003).
Uchigashima, M. et al. Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus, J. Neurosci. 27, 8088–8100 (2007).
Hendry, S. H. & Jones, E. G. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature 320, 750–753 (1986). This paper revealed activity-dependent neurotransmitter respecification in the adult primate brain.
Hendry, S. H. & Jones, E. G. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron 1, 701–712 (1988).
Perlstein, W. M., Carter, C. S., Noll, D. C. & Cohen, J. D. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am. J. Psychiatry 158, 1105–1113 (2001).
Akbarian, S. et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry 52, 258–266 (1995).
Brunelli, G. et al. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc. Natl Acad. Sci. USA 102, 8752–8757 (2005). This study illustrated the plasticity of neurotransmitter receptor expression at the adult mammalian neuromuscular junction.
Hirsch, E., Graybiel, A. M. & Agid, Y. A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature 334, 345–348 (1988).
Hartmann, A. et al. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc. Natl Acad. Sci. USA 97, 2875–2880 (2000).
Whitehouse, P. J. et al. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126 (1981).
Kar, S., Slowikowski, S. P., Westaway, D. & Mount, H. T. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J. Psychiatry Neurosci. 29, 427–441 (2004).
Muddashetty, R. S. et al. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 27, 5338–5348 (2007).
Nakamoto, M. et al. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc. Natl Acad. Sci. USA 104, 15537–15542 (2007).
Lam, R. W. & Levitan, R. D. Pathophysiology of seasonal affective disorder: a review. J. Psychiatry Neurosci. 25, 469–480 (2000).
Lam, R. W. et al. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am. J. Psychiatry 163, 805–812 (2006).
Trevino, M. & Gutierrez, R. The GABAergic projection of the dentate gyrus to hippocampal area CA3 of the rat: pre- and postsynaptic actions after seizures. J. Physiol. 567, 939–949 (2005).
Trevino, M., Vivar, C. & Gutierrez, R. β/γ oscillatory activity in the CA3 hippocampal area is depressed by aberrant GABAergic transmission from the dentate gyrus after seizures. J. Neurosci. 27, 251–259 (2007).
Tandé, D. et al. New striatal dopamine neurons in MPTP-treated macaques result from a phenotypic shift and not neurogenesis. Brain 129, 1194–1200 (2006).
Aumann, T. D. et al. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Exp. Neurol. 213, 419–430 (2008).
Patt, S., Gertz, H. J., Gerhard, L. & Cervós-Navarro, J. Pathological changes in dendrites of substantia nigra neurons in Parkinson's disease: a Golgi study. Histol. Histopathol. 6, 373–380 (1991).
Gerfen, C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311, 461–464 (1984).
Gerfen, C. R., Herkenham, M. & Thibault, J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci. 7, 3915–3934 (1987).
Hontanilla B, de las Heras, S. & Giménez-Amaya, J. M. A topographic re-evaluation of the nigrostriatal projections to the caudate nucleus in the cat with multiple retrograde tracers. Neuroscience 72, 485–503 (1996).
Rodríguez, M. & González-Hernández, T. Electrophysiological and morphological evidence for a GABAergic nigrostriatal pathway. J. Neurosci. 19, 4682–4694 (1999).
Earhart, G. M. Dance as therapy for individuals with Parkinson disease. Eur. J. Phys. Rehabil. Med. 45, 231–238 (2009).
Mendez, J. A. et al. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J. Neurosci. 28, 6309–6318 (2008).
Dal Bo, G. et al. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience 156, 59–70 (2008).
O'Dowd, D. K., Ribera, A. B. & Spitzer, N. C. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J. Neurosci. 18, 792–805 (1988).
Lockery, S. R. & Spitzer, N. C. Reconstruction of action potential development from whole-cell currents of differentiating spinal neurons. J. Neurosci. 12, 2268–2287 (1992).
McCobb, D. P., Best, P. M. & Beam, K. G. The differentiation of excitability in embryonic chick limb motoneurons. J. Neurosci. 10, 2974–2984 (1990).
Baines, R. A. & Bate, M. Electrophysiological development of central neurons in the Drosophila embryo. J. Neurosci. 18, 4673–4683 (1998).
Corlew, R., Bosma, M. M. & Moody, W. J. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J. Physiol. 560, 377–390 (2004).
Moreno, R. L. & Ribera, A. B. Zebrafish motor neuron subtypes differ electrically prior to axonal outgrowth. J. Neurophysiol. 102, 2477–2484 (2009).
Espósito, M. S. et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 25, 10074–10086 (2005).
Bixby, J. L. & Spitzer, N. C. Early differentiation of vertebrate spinal neurons in the absence of voltage-dependent Ca2+ and Na+ influx. Dev. Biol. 106, 89–96 (1984).
Henderson, L. P., Smith, M. A. & Spitzer, N. C. The absence of calcium blocks impulse-evoked release of acetylcholine but not de novo formation of functional neuromuscular synaptic contacts in culture. J. Neurosci. 4, 3140–3150 (1984).
Holliday, J. & Spitzer, N. C. Spontaneous calcium influx and its roles in differentiation of spinal neurons in culture. Dev. Biol. 141, 13–23 (1990).
Gu, X. & Spitzer, N. C. Low-threshold Ca2+ current and its role in spontaneous elevations of intracellular Ca2+ in developing Xenopus neurons. J. Neurosci. 13, 4936–4948 (1993).
Gu, X., Olson, E. C. & Spitzer, N. C. Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 14, 6325–6335 (1994).
Walicke, P. A., Campenot, R. B. & Patterson, P. H. Determination of transmitter function by neuronal activity. Proc. Natl Acad. Sci. USA 74, 5767–5771 (1977).
Sun, Y. A. & Poo, M. M. Evoked release of acetylcholine from the growing embryonic neuron. Proc. Natl Acad. Sci. USA 84, 2540–2544 (1987).
Hartenstein, V. Early pattern of neuronal differentiation in the Xenopus embryonic brainstem and spinal cord. J. Comp. Neurol. 328, 213–231 (1993).
Spitzer, N. C. & Borodinsky, L. N. Implications of activity-dependent neurotransmitter-receptor matching. Phil. Trans. R. Soc. B 363, 1393–1399 (2008).
Acknowledgements
I thank L. Borodinsky, D. Dulcis and M. Demarque for their comments and suggestions for the manuscript. N.C.S. is supported by grants NS15918 and NS57690 from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Activity-dependent neurotransmitter respecification
-
Expression or loss of a transmitter or switch between expression of one transmitter and another in response to changes in activity.
Rights and permissions
About this article
Cite this article
Spitzer, N. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci 13, 94–106 (2012). https://doi.org/10.1038/nrn3154
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3154
This article is cited by
-
A conserved transcriptional fingerprint of multi-neurotransmitter neurons necessary for social behavior
BMC Genomics (2022)
-
High-frequency stimulation of the subthalamic nucleus induces a sustained inhibition of serotonergic system via loss of cell phenotype
Scientific Reports (2022)
-
Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster
BMC Developmental Biology (2018)
-
A pupal transcriptomic screen identifies Ral as a target of store-operated calcium entry in Drosophila neurons
Scientific Reports (2017)
-
Lmx1b is required for the glutamatergic fates of a subset of spinal cord neurons
Neural Development (2016)