Abstract
There is a pressing need for treatments for neurodegenerative diseases. Hopes have been raised by the prospect of neural stem cell therapy; however, despite intense research activities and media attention, stem cell therapy for neurological disorders is still a distant goal. Effective strategies must be developed to isolate, enrich and propagate homogeneous populations of neural stem cells, and to identify the molecules and mechanisms that are required for their proper integration into the injured brain. This article examines these requirements, discusses the results obtained so far, and considers the steps that need to be taken to provide instruction to donor cells and to elucidate the neurogenic potential of the adult central nervous system environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Björklund, A. & Lindvall, O. Cell replacement therapies for central nervous system disorders. Nature Neurosci. 3, 537–544 (2000).
Bachoud-Levi, A. C. et al. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation. Lancet 356, 1945–1946 (2000).
Freeman, T. B. et al. Transplanted fetal striatum in Huntington disease: phenotypic development and lack of pathology. Proc Natl Acad Sci U S A 97, 13877–13882 (2000).
Temple, S. Stem cell plasticity — building the brain of our dreams. Nature Rev. Neurosci. 2, 513–520 (2000).
Blakemore, W. F., Smith, P. M. & Franklin, R. J. Remyelinating the demyelinated CNS. Novartis Found. Symp. 231, 289–298 (2000).
Brüstle, O. et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 285, 754–756 (1999).
Liu, S. et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl Acad. Sci. USA 97, 6126–6131 (2000).
Imaizumi, T., Lankford, K. L., Waxman, S. G., Greer, C. A. & Kocsis, J. D. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in demyelinated dorsal columns of the rat spinal cord. J. Neurosci. 18, 6176–6185 (1998).
Kohama, I. et al. Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. J. Neurosci. 21, 944–950 (2001).
Jeffery, N. D., Crang, A. J., O' Leary, M. T., Hodge, S. J. & Blakemore, W. F. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur. J. Neurosci. 11, 1508–1514 (1999).
Piccini, P. et al. Dopamine release from nigral transplants visualised in vivo in a Parkinson patient. Nature Neurosci. 2, 1137–1140 (1999).
Sotelo, C. & Alvarado-Mallart, R. M. The reconstruction of cerebellar circuits. Trends Neurosci. 14, 350–355 (1991).
Zhang, W., Lee, W. H. & Triarhou, L. C. Grafted cerebellar cells in a mouse model of hereditary ataxia express IGF-I system genes and partially restore behavioral function. Nature Med. 2, 65–71 (1996).
Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Kendall, A. L. et al. Functional integration of striatal allografts in a primate model of Huntington's disease. Nature Med. 4, 727–729 (1998).
Palfi, S. P. et al. Fetal striatal allografts reverse cognitive deficits in a primate model of Huntington disease. Nature Med. 4, 963–966 (1998).
Strata, P. & Rossi, F. Plasticity of the olivocerebellar pathway. Trends Neurosci. 21, 407–413 (1998).
Rossi, F., Saggiorato, C. & Strata, P. Target-specific innervation of cerebellar transplants by regenerating olivocerebellar axons in the adult rat. Exp. Neurol. 173, 205–212 (2002).
Grabowski, M., Brundin, P. & Johansson, B. B. Fetal neocortical grafts implanted in adult hypertensive rats with cortical infarcts following a middle cerebral artery occlusion: ingrowth of afferent fibers from the host brain. Exp. Neurol. 116, 106–121 (1992).
Grabowski, M., Johansson, B. B. & Brundin, P. Neocortical grafts placed in the infarcted brain of adult rats: few or no efferent fibers grow from transplant to host. Exp. Neurol. 134, 273–276 (1995).
Mattson, B., Sorensen, J. C., Zimmer, J. & Johansson, B. B. Neural grafting to experimental neocortical infarcts improves behavioural outcome and reduces thalamic atrophy in rats housed in enriched but not in standard environments. Stroke 28, 1225–1231 (1997).
Piccini, P. et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann. Neurol. 48, 689–695 (2000).
Döbrössy, M. D. & Dunnett, S. B. The influence of environment and experience on neural grafts. Nature Rev. Neurosci. 2, 871–879 (2001).
Brundin, P. et al. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 9, 179–195 (2000).
Schierle, G. S. et al. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nature Med. 5, 97–100 (1999).
Morrison, S. J., White, P. M., Zock, C. & Anderson, D. J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96, 737–749 (1999).
Rietze, R. L. et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature 412, 736–739 (2001).
Keyoung, H. L. et al. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nature Biotechnol. 19, 843–850 (2001).
Morrison, S. J. Neuronal potential and lineage determination by neural stem cells. Curr. Opin. Cell Biol. 13, 666–672 (2001).
Temple, S. The development of neural stem cells. Nature 414, 112–117 (2002).
Zappone, M. V. et al. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127, 2367–2382 (2000).
Hitoshi, S., Tropepe, V., Ekker, M. & Van der Kooy, D. Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain. Development 129, 233–244 (2002).
Winkler, C. et al. Incorporation and glial differentiation of mouse EGF-responsive neural progenitor cells after transplantation into the embryonic rat brain. Mol. Cell. Neurosci. 11, 99–116 (1998).
Svendsen, C. N. et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp. Neurol. 148, 135–146 (1997).
Cao, Q. et al. Pluripotent stem cells engrafted in the normal or lesioned adult rat spinal cord are restricted to a glial cell lineage. Exp. Neurol. 167, 48–58 (2001).
Chow, S. I. et al. Characterization and intraspinal grafting of EGF/bFGF-dependent neurospheres derived from embryonic rat spinal cord. Brain Res. 874, 87–106 (2000).
Morshead, C. M., Benveniste, P., Iscove, N. N. & Van der Kooy, D. Hematopoietic competence is a rare property of neural stem cells and may depend on genetic and epigenetic alterations. Nature Med. 8, 268–273 (2002).
Studer, L. et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 20, 7377–7383 (2000).
Zetterström, R. H. et al. Dopamine neuron agenesis in Nurr-1-deficient mice. Science 276, 248–250 (1997).
Wagner, J. et al. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nature Biotechnol. 17, 653–659 (1999).
Sakurada, K., Oshimo-Sakurada, M., Palmer, T. D. & Gage, F. H. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development 126, 4017–4026 (1999).
Bonni, A. et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278, 477–483 (1997).
Conti, L. et al. Expression and activation of SH2/PTB-containing ShcA adapter protein reflects the pattern of neurogenesis in the mammalian brain. Proc. Natl Acad. Sci. USA 94, 8185–8190 (1997).
Cattaneo, E. & Pelicci, P. G. Emerging roles for SH2/PTB-containing Shc adapter proteins in the developing mammalian brain. Trends Neurosci. 21, 476–481 (1998).
Conti, L. et al. Shc signaling in differentiating neural progenitor cells. Nature Neurosci. 4, 579–586 (2001).
Sun, Y. et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104, 365–376 (2001).
Bjornson, C. R. R., Rietze, R. L., Reynolds, B. A., Magli, L. C. & Vescovi, A. L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283, 534–537 (1999).
Brazelton, T. R., Rossi, F. M. V., Keshet, G. I. & Blau, H. M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290, 1775–1779 (2000).
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290, 1779–1782 (2000).
Clarke, D. L. et al. Generalized potential of adult neural stem cells. Science 288, 1660–1663 (2000).
Ying, Q.-L., Nichols, J., Ewans, E. P. & Smith, A. G. Changing potency by spontaneous fusion. Nature 416, 545–548 (2002).
Terada, N. et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542–545 (2002).
McKinney-Freeman, S. L. et al. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc. Natl Acad. Sci. USA 99, 1341–1346 (2002).
Toma, J. G. et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell Biol. 3, 778–784 (2001).
Brüstle, O., Maskos, U. & McKay, R. D. G. Host-guided migration allows targeted introduction of neurons into the embryonic brain. Neuron 15, 1275–1285 (1995).
Campbell, K., Olsson, M. & Björklund, A. Regional incorporation and site-specific differentiation of striatal precursors transplanted to the embryonic forebrain ventricle. Neuron 15, 1259–1273 (1995).
Lim, D. A., Fishell, G. A. & Alvarez-Buylla, A. Postnatal mouse subventricular zone neuronal precursors can migrate and differentiate within multiple levels of the developing neuraxis. Proc. Natl Acad. Sci. USA 94, 14832–14836 (1997).
Magrassi, L. et al. Basal ganglia precursors found in aggregates following embryonic transplantation adopt a striatal phenotype in heterotopic locations. Development 125, 2847–2855 (1998).
Gage, F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000).
Gould, E., Reeves, A. J., Graziano, M. S. A. & Gross, C. G. Neurogenesis in the neocortex of adult primates. Science 286, 548–552 (1999).
Kornack, D. R. & Rakic, P. Cell proliferation without neurogenesis in the adult primate neocortex. Science 294, 2127–2130 (2001).
Suhonen, J. O., Peterson, D. A., Ray, J. & Gage, F. H. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383, 624–627 (1996).
Shihabuddin, L. S., Horner, P. J., Ray, J. & Gage, F. H. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 20, 8727–8735 (2000).
Herrera, D. G., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann. Neurol. 46, 867–877 (1999).
Lim, D. A. et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726 (2000).
Renfranz, P. J., Cunningham, M. G. & McKay, R. D. G. Region-specific differentiation of the hippocampal cell line HiB5 upon implantation into the developing mammalian brain. Cell 66, 713–729 (1991).
Snyder, E. Y. et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68, 33–51 (1992).
Gao, W.-Q. & Hatten, M. E. Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development 120, 1059–1070 (1994).
Shihabuddin, L. S., Hertz, J. A., Holets, V. R. & Whittemore, S. R. The adult CNS retains the potential to direct region-specific differentiation of a transplanted neuronal precursor cell line. J. Neurosci. 15, 6666–6678 (1995).
Lundberg, C., Englund, U., Trono, D., Björklund, A. & Wictorin, K. Differentiation of RN33B cell line into forebrain projection neurons after transplantation into the neonatal rat brain. Exp. Neurol. (in the press).
Catapano, L. A., Sheen, V. L., Leavitt, B. R. & Macklis, J. D. Differentiation of transplanted neural precursors varies regionally in adults striatum. Neuroreport 10, 3971–3977 (1999).
McEwen, B. S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122 (1999).
Gould, E., Tanapat, P., Rydel, T. & Hastings, N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry 48, 715–720 (2000).
Doetsch, F., Caillé, I., Lim, D. A., Garcia-Verdugo, L. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Jin, K. et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl Acad. Sci. USA 98, 4710–4715 (2001).
Jankovski, A., Garcia, C., Soriano, E. & Sotelo, C. Proliferation, migration and differentiation of neuronal progenitor cells in the adult mouse subventricular zone surgically separated from its olfactory bulb. Eur. J. Neurosci. 10, 3853–3868 (1998).
Gould, E. & Tanapat, P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience 80, 427–436 (1997).
Bengzon, J. et al. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc. Natl Acad. Sci. USA 94, 10432–10437 (1997).
Parent, J. M. et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 17, 3727–3738 (1997).
Blumcke, I. et al. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus 11, 311–321 (2001).
Yoshimura, S. et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc. Natl Acad. Sci. USA 98, 5874–5879 (2001).
Palmer, T. D., Markakis, E. A., Willhoite, A. R., Safar, F. & Gage, F. H. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 19, 8487–8497 (1999).
Marti, H. H. et al. Erythropoietin gene expression in human, monkey and murine brain. Eur. J. Neurosci. 8, 666–676 (1996).
Shingo, T., Sorokan, S. T., Shimazaki, T. & Weiss, S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J. Neurosci. 21, 9733–9743 (2001).
Magavi, S. S., Leavitt, B. R. & Macklis, J. D. Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955 (2000).
Macklis, J. D. Transplanted neocortical neurons migrate selectively in regions of neuronal degeneration produced by chromophore-targeted laser photolysis. J. Neurosci. 13, 3848–3863 (1993).
Snyder, E. Y., Yoon, C., Flax, J. D. & Macklis, J. D. Mulitpotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc. Natl Acad. Sci. USA 94, 11663–11668 (1997).
Priller, J. et al. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 155, 733–738 (2001).
Zigova, T. et al. Neuronal progenitor cells of the neonatal subventricular zone differentiate and disperse following transplantation into the adult rat striatum. Cell Transplant. 7, 137–156 (1998).
Gage, F. H. et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl Acad. Sci. USA 92, 11879–11883 (1995).
Shihabuddin, L. S., Holets, V. R. & Whittemore, S. R. Selective hippocampal lesions differentially affect the phenotypic fate of transplanted neuronal precursors. Exp. Neurol. 139, 61–72 (1996).
Lundberg, C., Winkler, C., Whittemore, S. R. & Björklund, A. Conditionally immortalised neural progenitor cells grafted to the striatum exhibit site-specific neuronal differentiation and establish connections with the host globus pallidus. Neurobiol. Dis. 3, 33–50 (1996).
Lundberg, C., Martinez-Serrano, A., Cattaneo, E., McKay, R. D. G. & Björklund, A. Survival, integration, and differentiation of neural stem cell lines after transplantation to the adult rat striatum. Exp. Neurol. 145, 342–360 (1997).
Björklund, L. M. et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl Acad. Sci. USA 99, 2344–2349 (2002).
Vallières, L., Campbell, I. L., Gage, F. H. & Sawchenko, P. E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 22, 486–492 (2002).
Craig, C. G. et al. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 16, 2649–2658 (1996).
Kuhn, G. H., Winkler, J., Kempermann, G., Thal, L. J. & Gage, F. H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 17, 5820–5829 (1997).
Benraiss, A., Chmielnicki, E., Lerner, K., Roh, D. & Goldman, S. A. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J. Neurosci. 21, 6718–6731 (2001).
Zigova, T., Pencea, V., Wiegand, S. J. & Luskin, M. B. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol. Cell. Neurosci. 11, 234–245 (1998).
Pencea, V., Bingaman, K. D., Wiegand, S. J. & Luskin, M. B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 21, 6706–6717 (2001).
Fallon, J. et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl Acad. Sci. USA 97, 14686–14691 (2000).
McLaren, A. Ethical and social considerations of stem cell research. Nature 414, 129–131 (2001).
Adam, D. Britain banks on embryonic stem cells to gain competitive edge. Nature 416, 3–4 (2002).
Terskikh, A. V. et al. From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs. Proc. Natl Acad. Sci. USA 98, 7934–7939 (2001).
Kornblum, H. I. & Geschwind, D. H. Molecular targets in CNS stem cell research: hitting a moving target. Nature Rev. Neurosci. 2, 843–846 (2001).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Smith, A. G. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435–462 (2001).
Eiges, R. et al. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr. Biol. 11, 514–518 (2001).
Reubinoff, B. E. et al. Neural progenitors from human embryonic stem cells. Nature Biotechnol. 19, 1134–1140 (2001).
Zhang, S. C., Wernig, M., Duncan, I. D., Brustle, O. & Thomson, J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnol. 19, 1129–1133 (2001).
Vogel, G. Stem cells. In the Mideast, pushing back the stem cell frontier. Science 295, 1818–1820 (2002).
Zuccato, C. et al. Loss of Huntingtin–mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001).
Acknowledgements
Our work is supported by grants from the Ministero dell'Istruzione, dell'Università e della Ricerca, the Ministero della Sanità-Progetto Alzheimer, Telethon, the Associazione Italiana Ricerca sul Cancro, the Huntington's Disease Society of America and the Hereditary Disease Foundation. We thank L. Conti, R. McKay, M. Peschanski and P. Strata for helpful comments. We apologize for the omission of several beautiful papers that could not be cited owing to space limitations.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
Rights and permissions
About this article
Cite this article
Rossi, F., Cattaneo, E. Neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci 3, 401–409 (2002). https://doi.org/10.1038/nrn809
Issue Date:
DOI: https://doi.org/10.1038/nrn809
This article is cited by
-
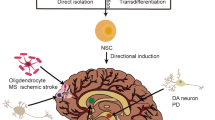
Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke
Cell Death Discovery (2023)
-
Application of Ultrasound to Enhancing Stem Cells Associated Therapies
Stem Cell Reviews and Reports (2023)
-
Nanoparticles Treat Ischemic Stroke by Responding to Stroke Microenvironment
BioNanoScience (2023)
-
Neuromodulation-Based Stem Cell Therapy in Brain Repair: Recent Advances and Future Perspectives
Neuroscience Bulletin (2021)
-
Neurospheres: a potential in vitro model for the study of central nervous system disorders
Molecular Biology Reports (2021)