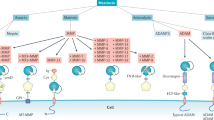
Abstract
The 50 kDa glycoprotein plasminogen activator inhibitor 1 (PAI-1) is the major physiological inhibitor of tissue-type and urokinase-type plasminogen activator. These two molecules convert inactive plasminogen into its fibrin-degrading form, plasmin. Plasma and tissue concentrations of PAI-1 are extremely low under normal circumstances but increase under pathologic conditions. This increase is mediated by many factors, including reactive oxygen species. Increased PAI-1 activity is associated with an increased risk of ischemic cardiovascular events and tissue fibrosis. Whereas the antifibrinolytic property of PAI-1 derives mainly from its inhibition of serine proteases, its profibrotic actions seem to derive from a capacity to stimulate interstitial macrophage recruitment and increase transcription of profibrotic genes, as well as from inhibition of serine proteases. Despite studies in mice that lack or overexpress PAI-1, the biological effects of this molecule in humans remain incompletely understood because of the complexity of the PAI-1–plasminogen-activator–plasmin system. The cardioprotective and renoprotective properties of some currently available drugs might be attributable in part to inhibition of PAI-1. The development of an orally active, high-affinity PAI-1 inhibitor will provide a potentially important pharmacological tool for further investigation of the role of PAI-1 and might offer a novel therapeutic strategy in renal and cardiovascular diseases.
Key Points
-
Plasminogen activator inhibitor 1 (PAI-1) is an antifibrinolytic and profibrotic protein; its antifibrinolytic properties derive mainly from inhibition of serine protease activity
-
The mechanisms of PAI-1's profibrotic action are complex; in addition to inhibiting serine proteases, PAI-1 might promote interstitial macrophage recruitment and exert direct cellular effects through binding the urokinase-type plasminogen activator receptor
-
Improved understanding of the signaling pathways involved in PAI-1-induced gene transcription will aid the development of novel therapeutic strategies for ischemic and fibrotic diseases
-
Several currently available renoprotective drugs have PAI-1 inhibitory activity; development of an orally active, specific PAI-1 inhibitor could provide a pharmacological tool for investigating the role of PAI-1 and might have therapeutic potential
-
Reactive oxygen species have a role in the upregulation of PAI-1; therefore, the efficacy of antioxidants in the treatment and prevention of ischemic cardiovascular disease and renal fibrosis warrants investigation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wiman, B. & Collen, D. Purification and characterization of human antiplasmin, the fast-acting plasmin inhibitor in plasma. Eur. J. Biochem. 78, 19–26 (1977).
Eddy, A. A. & Fogo, A. B. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J. Am. Soc. Nephrol 17, 2999–3012 (2006).
Liu, R. M. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid. Redox Signal. 10, 303–319 (2008).
Vaughan, D. E. et al. PAI-1 antagonists: predictable indications and unconventional applications. Curr. Drug Targets 8, 962–970 (2007).
Hoffstedt, J. et al. The common -675 4G/5G polymorphism in the plasminogen activator inhibitor-1 gene is strongly associated with obesity. Diabetologia 45, 584–587 (2002).
Lundgren, C. H. et al. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 93, 106–110 (1996).
Stefansson, S. et al. Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr. Pharm. Des. 9, 1545–1564 (2003).
Myohanen, H. & Vaheri, A. Regulation and interactions in the activation of cell-associated plasminogen. Cell. Mol. Life Sci. 61, 2840–2858 (2004).
Cesarman-Maus, G. & Hajjar, K. A. Molecular mechanisms of fibrinolysis. Br. J. Haematol. 129, 307–321 (2005).
Cale, J. M. & Lawrence, D. A. Structure–function relationships of plasminogen activator inhibitor-1 and its potential as a therapeutic agent. Curr. Drug Targets 8, 971–981 (2007).
Andreotti, F. et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am. J. Cardiol. 62, 635–637 (1988).
Angleton, P. et al. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 79, 101–106 (1989).
Muller, J. E. et al. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79, 733–743 (1989).
Kurnik, P. B. Circadian variation in the efficacy of tissue-type plasminogen activator. Circulation 91, 1341–1346 (1995).
Massague, J. The transforming growth factor-β family. Annu. Rev. Cell Biol. 6, 597–641 (1990).
Irigoyen, J. P. et al. The plasminogen activator system: biology and regulation. Cell. Mol. Life Sci. 56, 104–132 (1999).
Bosma, P. J. et al. Human plasminogen activator inhibitor-1 gene. Promoter and structural gene nucleotide sequence. J. Biol. Chem. 263, 9129–9141 (1988).
Klinger, K. W. et al. Plasminogen activator inhibitor type 1 gene is located at region q21.3–q22 of chromosome 7 and genetically linked with cystic fibrosis. Proc. Natl Acad. Sci. USA 84, 8548–8552 (1987).
Bruzdzinski, C. J. et al. Isolation and characterization of the rat plasminogen activator inhibitor-1 gene. J. Biol. Chem. 265, 2078–2085 (1990).
Prendergast, G. C. et al. The c-myc-regulated gene mrl encodes plasminogen activator inhibitor 1. Mol. Cell Biol. 10, 1265–1269 (1990).
Keeton, M. R. et al. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J. Biol. Chem. 266, 23048–23052 (1991).
Dawson, S. J. et al. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J. Biol. Chem. 268, 10739–10745 (1993).
Chen, Y. Q. et al. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J. Biol. Chem. 273, 8225–8231 (1998).
Dennler, S. et al. Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17, 3091–3100 (1998).
Fink, T. et al. Identification of a tightly regulated hypoxia-response element in the promoter of human plasminogen activator inhibitor-1. Blood 99, 2077–2083 (2002).
Dawson, S. et al. Genetic variation at the plasminogen activator inhibitor-1 locus is associated with altered levels of plasma plasminogen activator inhibitor-1 activity. Arterioscler. Thromb. Vasc. Biol. 11, 183–190 (1991).
Eriksson, P. et al. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc. Natl Acad. Sci. USA 92, 1851–1855 (1995).
Lane, D. A. & Grant, P. J. Role of hemostatic gene polymorphism in venous and arterial thrombotic disease. Blood 95, 1517–1532 (2000).
Wong, T. Y. et al. Association of plasminogen activator inhibitor-1 4G/4G genotype and type 2 diabetic nephropathy in Chinese patients. Kidney Int. 57, 632–638 (2000).
Lee, E. A. et al. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 67, 1762–1771 (2005).
Jiang, Z. et al. Reactive oxygen species mediate TGF-β1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem. Biophys. Res. Commun. 309, 961–966 (2003).
Liao, H. et al. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1α, and C/EBPα. FASEB J. 21, 935–949 (2007).
Sakamoto, T. et al. TNF-α and insulin, alone and synergistically, induce plasminogen activator inhibitor-1 expression in adipocytes. Am. J. Physiol. 276, C1391–C1397 (1999).
Yoshimoto, T. et al. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology 145, 3331–3337 (2004).
Yoshimoto, T. et al. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens. Res. 28, 165–172 (2005).
Vayalil, P. K. et al. Glutathione suppresses TGF-β-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L1281–L1292 (2007).
Rhyu, D. Y. et al. Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 16, 667–675 (2005).
Manna, S. K. et al. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-κB and activated protein-1. J. Biol. Chem. 273, 13245–13254 (1998).
Brown, N. J. et al. Modulation of angiotensin II and norepinephrine-induced plasminogen activator inhibitor-1 expression by AT1a receptor deficiency. Kidney Int. 72, 72–81 (2007).
Brown, N. J. et al. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int. 58, 1219–1227 (2000).
Brown, N. J. et al. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J. Clin. Endocrinol. Metab. 85, 336–344 (2000).
Ma, L. J. et al. TGF-β dependent and –independent pathways of induction of tubulointerstitial fibrosis in β6−/− mice. Am. J. Pathol. 163, 1261–1273 (2003).
Goff, D. C., Jr et al. Essential features of a surveillance system to support the prevention and management of heart disease and stroke: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Stroke, and Cardiovascular Nursing and the Interdisciplinary Working Groups on Quality of Care and Outcomes Research and Atherosclerotic Peripheral Vascular Disease. Circulation 115, 127–155 (2007).
Ford, E. S. et al. Sedentary behavior, physical activity, and the metabolic syndrome among, U.S. adults. Obes. Res. 13, 608–614 (2005).
Erickson, L. A. et al. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nature 346, 74–76 (1990).
Eren, M. et al. Age-dependent spontaneous coronary arterial thrombosis in transgenic mice that express a stable form of human plasminogen activator inhibitor-1. Circulation 106, 491–496 (2002).
Peng, L. et al. Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler. Thromb. Vasc. Biol. 22, 934–939 (2002).
Carmeliet, P. et al. Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation: a gene targeting and gene transfer study in mice. Circulation 96, 3180–3191 (1997).
Otsuka, G. et al. Transforming growth factor β1 induces neointima formation through plasminogen activator inhibitor-1-dependent pathways. Arterioscler. Thromb. Vasc. Biol. 26, 737–743 (2006).
de Waard, V. et al. Plasminogen activator inhibitor 1 and vitronectin protect against stenosis in a murine carotid artery ligation model. Arterioscler. Thromb. Vasc. Biol. 22, 1978–1983 (2002).
Lijnen, H. R. et al. Neointima formation and thrombosis after vascular injury in transgenic mice overexpressing plasminogen activator inhibitor-1 (PAI-1). J. Thromb. Haemost. 2, 16–22 (2004).
Alexander, C. M. et al. Proteinases and extracellular matrix remodeling. Curr. Opin. Cell Biol. 1, 974–982 (1989).
Stefansson, S. et al. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature 383, 441–443 (1996).
Chen, Y. et al. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler. Thromb. Vasc. Biol. 26, 1777–1783 (2006).
Kwaan, H. C. et al. Plasminogen activator inhibitor 1 may promote tumour growth through inhibition of apoptosis. Br. J. Cancer 82, 1702–1708 (2000).
Fay, W. P. Plasminogen activator inhibitor 1, fibrin, and the vascular response to injury. Trends Cardiovasc. Med. 14, 196–202 (2004).
Sjoland, H. et al. Atherosclerosis progression in LDL receptor-deficient and apolipoprotein E-deficient mice is independent of genetic alterations in plasminogen activator inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 20, 846–852 (2000).
Eitzman, D. T. et al. Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood 96, 4212–4215 (2000).
Luttun, A. et al. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 22, 499–505 (2002).
Prisco, D. et al. Postprocedural PAI-1 activity is a risk marker of subsequent clinical restenosis in patients both with and without stent implantation after elective balloon PTCA. Thromb. Res. 104, 181–186 (2001).
Strauss, B. H. et al. Plasma urokinase antigen and plasminogen activator inhibitor-1 antigen levels predict angiographic coronary restenosis. Circulation 100, 1616–1622 (1999).
Christ, G. et al. Predictive value of plasma plasminogen activator inhibitor-1 for coronary restenosis: dependence on stent implantation and antithrombotic medication. J. Thromb. Haemost. 3, 233–239 (2005).
Rondeau, E. et al. Plasminogen activator inhibitor 1 in renal fibrin deposits of human nephropathies. Clin. Nephrol. 33, 55–60 (1990).
Nakamura, T. The localization of plasminogen activator inhibitor-1 in glomerular subepithelial deposits in membranous nephropathy. J. Am. Soc. Nephrol. 7, 2434–2444 (1996).
Hamano, K. et al. Expression of glomerular plasminogen activator inhibitor type I in glomerulonephritis. Am. J. Kidney Dis. 39, 695–705 (2002).
Paueksakon, P. et al. Microangiopathic injury and augmented PAI-1 in human diabetic nephropathy. Kidney Int. 61, 2142–2148 (2002).
Gesualdo, L. et al. Angiotensin IV stimulates plasminogen activator inhibitor-1 expression in proximal tubular epithelial cells. Kidney Int. 56, 461–470 (1999).
Matsuo, S. et al. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int. 67, 2221–2238 (2005).
Haraguchi, M. et al. t-PA promotes glomerular plasmin generation and matrix degradation in experimental glomerulonephritis. Kidney Int. 59, 2146–2155 (2001).
Lee, H. B. et al. Suppression of plasminogen activator inhibitor-1 inhibits high glucose- and TGF-β1-induced fibronectin secretion and increases MMP2 activity by mesnagial cells [Abstract]. J. Am. Soc. Nephrol. 13, 169A (2002).
Oda, T. et al. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 60, 587–596 (2001).
Kitching, A. R. et al. Plasminogen activator inhibitor-1 is a significant determinant of renal injury in experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 14, 1487–1495 (2003).
Huang, Y. et al. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J. Clin. Invest. 112, 379–388 (2003).
Ma, L. J. et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 53, 336–346 (2004).
Collins, S. J. et al. Plasminogen activator inhibitor-1 deficiency has renal benefits but some adverse systemic consequences in diabetic mice. Nephron Exp. Nephrol. 104, e23–e34 (2006).
Yang, J. et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Invest. 110, 1525–1538 (2002).
Nicholas, S. B. et al. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 67, 1297–1307 (2005).
Lyons, R. M. et al. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J. Cell Biol. 110, 1361–1367 (1990).
Hertig, A. et al. Type 1 plasminogen activator inhibitor deficiency aggravates the course of experimental glomerulonephritis through overactivation of transforming growth factor beta. FASEB J. 17, 1904–1906 (2003).
Edgtton, K. L. et al. Plasmin is not protective in experimental renal interstitial fibrosis. Kidney Int. 66, 68–76 (2004).
Preissner, K. T. et al. Urokinase receptor: a molecular organizer in cellular communication. Curr. Opin. Cell Biol. 12, 621–628 (2000).
Degryse, B. et al. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J. Biol. Chem. 279, 22595–22604 (2004).
Nykjaer, A. et al. Regions involved in binding of urokinase-type-1 inhibitor complex and pro-urokinase to the endocytic α2-macroglobulin receptor/low density lipoprotein receptor-related protein. Evidence that the urokinase receptor protects pro-urokinase against binding to the endocytic receptor. J. Biol. Chem. 269, 25668–25676 (1994).
Biemond, B. J. et al. Thrombolysis and reocclusion in experimental jugular vein and coronary artery thrombosis. Effects of a plasminogen activator inhibitor type 1-neutralizing monoclonal antibody. Circulation 91, 1175–1181 (1995).
van Giezen, J. J. et al. The Fab-fragment of a PAI-1 inhibiting antibody reduces thrombus size and restores blood flow in a rat model of arterial thrombosis. Thromb. Haemost. 77, 964–969 (1997).
Fay, W. P. et al. Human plasminogen activator inhibitor-1 (PAI-1) deficiency: characterization of a large kindred with a null mutation in the PAI-1 gene. Blood 90, 204–208 (1997).
Izuhara, Y. et al. Inhibition of plasminogen activator inhibitor-1: its mechanism and effectiveness on coagulation and fibrosis. Arterioscler. Thromb. Vasc. Biol. 28, 672–677 (2008).
Leik, C. E. et al. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J. Thromb. Haemost. 4, 2710–2715 (2006).
Gorlatova, N. V. et al. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. J. Biol. Chem. 282, 9288–9296 (2007).
Elokdah, H. et al. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. J. Med. Chem. 47, 3491–3494 (2004).
Hennan, J. K. et al. Evaluation of PAI-039 [{1-benzyl-5-[4-(trifluoromethoxy)phenyl]-1H-indol-3-yl}(oxo)acetic acid], a novel plasminogen activator inhibitor-1 inhibitor, in a canine model of coronary artery thrombosis. J. Pharmacol. Exp. Ther. 314, 710–716 (2005).
Weisberg, A. D. et al. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler. Thromb. Vasc. Biol. 25, 365–371 (2005).
Crandall, D. L. et al. Modulation of adipose tissue development by pharmacological inhibition of PAI-1. Arterioscler. Thromb. Vasc. Biol. 26, 2209–2215 (2006).
Liang, A. et al. Characterization of a small molecule PAI-1 inhibitor, ZK4044. Thromb. Res. 115, 341–350 (2005).
Crandall, D. L. et al. WAY-140312 reduces plasma PAI-1 while maintaining normal platelet aggregation. Biochem. Biophys. Res. Commun. 311, 904–908 (2003).
Vaughan, D. E. et al. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. HEART Study Investigators. Circulation 96, 442–447 (1997).
Fogari, R. & Zoppi, A. Antihypertensive drugs and fibrinolytic function. Am. J. Hypertens. 19, 1293–1299 (2006).
Liu, N. et al. Angiotensin II receptor blockade ameliorates mesangioproliferative glomerulonephritis in rats through suppression of CTGF and PAI-1, independently of the coagulation system. Nephron Exp. Nephrol. 105, e65–e74 (2007).
Oikawa, T. et al. Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. 51, 164–172 (1997).
Skurk, T. & Hauner, H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int. J. Obes. Relat. Metab. Disord. 28, 1357–1364 (2004).
Hoo, R. L. et al. Adiponectin mediates the suppressive effect of rosiglitazone on plasminogen activator inhibitor-1 production. Arterioscler. Thromb. Vasc. Biol. 27, 2777–2782 (2007).
Zambrana, J. L. et al. Comparison of bezafibrate versus lovastatin for lowering plasma insulin, fibrinogen, and plasminogen activator inhibitor-1 concentrations in hyperlipemic heart transplant patients. Am. J. Cardiol. 80, 836–840 (1997).
Song, C. Y. et al. Lovastatin inhibits oxidized low-density lipoprotein-induced plasminogen activator inhibitor and transforming growth factor-β1 expression via a decrease in Ras/extracellular signal-regulated kinase activity in mesangial cells. Transl. Res. 151, 27–35 (2008).
Wang, L. et al. Simvastatin reduces circulating plasminogen activator inhibitor 1 activity in volunteers with the metabolic syndrome. Metab. Syndr. Relat. Disord. 6, 149–152 (2008).
Grafe, M. et al. Human cardiac microvascular and macrovascular endothelial cells respond differently to oxidatively modified LDL. Atherosclerosis 137, 87–95 (1998).
Bonfigli, A. R. et al. Vitamin E intake reduces plasminogen activator inhibitor type 1 in T2DM patients. Diabetes Nutr. Metab. 14, 71–77 (2001).
Uchida, Y. et al. Cellular carbonyl stress enhances the expression of plasminogen activator inhibitor-1 in rat white adipocytes via reactive oxygen species-dependent pathway. J. Biol. Chem. 279, 4075–4083 (2004).
Marx, N. et al. PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARγ as a potential mediator in vascular disease. Arterioscler. Thromb. Vasc. Biol. 19, 546–551 (1999).
Acknowledgements
This work was supported in part by grants from the Korea Research Foundation (#E00014), the Korea Science and Engineering Foundation (#ROI-2006-000-10829-0 and #R15-2006-020), and the second stage of Brain Korea 21 Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ha, H., Oh, E. & Lee, H. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol 5, 203–211 (2009). https://doi.org/10.1038/nrneph.2009.15
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.15
This article is cited by
-
Unveiling the molecular Hallmarks of Peyronie’s disease: a comprehensive narrative review
International Journal of Impotence Research (2024)
-
COVID-19: spot urine rather than bronchoalveolar lavage fluid analysis?
Critical Care (2021)
-
Immunoreactivity of Plasminogen Activator Inhibitor 1 and Its Correlation with Dysmenorrhea and Lesional Fibrosis in Adenomyosis
Reproductive Sciences (2021)
-
Intracellular high cholesterol content disorders the clock genes, apoptosis-related genes and fibrinolytic-related genes rhythmic expressions in human plaque-derived vascular smooth muscle cells
Lipids in Health and Disease (2017)
-
MicroRNAs in kidney physiology and disease
Nature Reviews Nephrology (2015)