Abstract
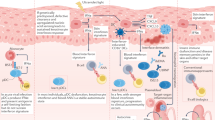
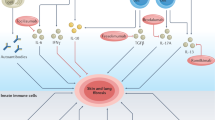
Transforming growth factor β (TGF-β) is a pleiotropic cytokine with vital homeostatic functions. Aberrant TGF-β expression is implicated in the pathogenesis of fibrosis in systemic sclerosis (SSc); thus, TGF-β represents a molecular therapeutic target in this disease. Anti-TGF-β monoclonal antibody has been evaluated in a small trial of early SSc, with disappointing results. Antibodies against the αvβ6 integrin that prevent latent TGF-β activation, however, have shown promise in preclinical studies. Small-molecule inhibitors of TGF-β-receptor activity are effective in animal models of fibrosis. Imatinib mesylate and related tyrosine kinase inhibitors also block TGF-β pathways and abrogate fibrotic responses. The blocking of TGF-β activity might lead to spontaneous immune activation, epithelial hyperplasia and impaired wound healing. Loss of immune tolerance is a potential concern in an autoimmune disease such as SSc. Novel insights from microarray-based gene expression analyses and studies of genetic polymorphisms in TGF-β signaling could aid in identifying patients who are most likely to respond to anti-TGF-β treatment. This intervention promises to have a major impact on the treatment of SSc. Concerns regarding efficacy and safety and whether biomarkers can indicate these features, questions regarding appropriate dosing and timing of therapy, and identification of potential responders are critical challenges ahead.
Key Points
-
Systemic sclerosis (SSc) is a highly heterogeneous fibrotic condition that has no effective disease-modifying therapy; arresting disease progression and reversing organ damage will require antifibrotic therapies
-
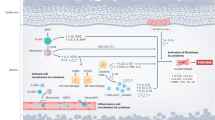
Fibrosis is associated with fibroblast activation mediated by transforming growth factor β (TGF-β); therefore, blocking TGF-β signaling pathways is a rational approach to antifibrotic therapy
-
Targeting the TGF-β pathway with biologic therapies prevents fibrosis in animal models, but efficacy has not yet been shown in patients with SSc
-
Small-molecule inhibitors of tyrosine kinases block TGF-β signaling and prevent TGF-β-driven fibrotic responses in vitro and in vivo; clinical trials are evaluating two such inhibitors in patients with SSc
-
Blocking the TGF-β pathway could be associated with adverse effects such as loss of immune tolerance and spontaneous autoimmunity, epithelial hyperplasia, and defective tissue repair
-
Key challenges for the development of anti-TGF-β therapies in SSc include determining optimum timing and dosing, and developing biomarkers of biological response and clinical efficacy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Varga, J. & Abraham, D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 (2007).
Blobe, G. C. et al. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342, 1350–1358 (2000).
Prud'homme, G. J. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Invest. 87, 1077–1091 (2007).
Feng, X. H. & Derynck, R. Specificity and versatility in TGF-beta signaling through SMADs. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005).
Gordon, K. J. & Blobe, G. C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 1782, 197–228 (2008).
Valle, L. et al. Germline allele-specific expression of TGFBR1 confers an increased risk of colorectal cancer. Science 321, 1361–1365 (2008).
Zeng, Q. et al. TGFBR1 haploinsufficiency is a potent modifier of colorectal cancer development. Cancer Res. 69, 678–686 (2009).
Saxena, V. et al. Dual roles of immunoregulatory cytokine TGF-beta in the pathogenesis of autoimmunity-mediated organ damage. J. Immunol. 180, 1903–1912 (2008).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Sonnylal, S. et al. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 56, 334–344 (2007).
Wu, M. & Varga, J. In perspective: murine models of scleroderma. Curr. Rheumatol. Rep. 10, 173–182 (2008).
Whitfield, M. L. et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl Acad. Sci. USA 100, 12319–12324 (2003).
Milano, A. et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 3, e2696 (2008).
Annes, J. P. et al. Making sense of latent TGF-β activation. J. Cell Sci. 116, 217–224 (2003).
Varga, J. Scleroderma and SMADs: dysfunctional SMAD family dynamics culminating in fibrosis. Arthritis Rheum. 46, 1703–1713 (2002).
Rahimi, R. A. & Leof, E. B. TGF-beta signaling: a tale of two responses. J. Cell Biochem. 102, 593–608 (2007).
Derynck, R. & Zhang, Y. E. SMAD-dependent and SMAD-independent pathways in TGF-beta family signalling. Nature 425, 577–584 (2003).
Lin, X. et al. PPM1A functions as a SMAD phosphatase to terminate TGF-β signaling. Cell 125, 915–928 (2006).
Lin, F. et al. MAN1, an integral protein of the inner nuclear membrane, binds SMAD2 and SMAD3 and antagonizes transforming growth factor-beta signaling. Hum. Mol. Genet. 14, 437–445 (2005).
Di Guglielmo, G. M. et al. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 5, 410–421 (2003).
Galdo, F. D. et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 58, 2854–2865 (2008).
Tourkina, E. et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L843–L861 (2008).
Ziyadeh, F. N. et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal anti-transforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl Acad. Sci. USA 97, 8015–8020 (2000).
McCormick, L. L. et al. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J. Immunol. 163, 5693–5699 (1999).
Fukasawa, H. et al. Treatment with anti-TGF-beta antibody ameliorates chronic progressive nephritis by inhibiting SMAD/TGF-beta signaling. Kidney Int. 65, 63–74 (2004).
Denton, C. P. et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 56, 323–333.
Mead, A. L. et al. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest. Ophthalmol. Vis. Sci. 44, 3394–3401 (2003).
ClinicalTrials.gov [http://clinicaltrials.gov/ct/show/NCT00125385] (accessed 20 January 2009).
Juárez, P. et al. Soluble betaglycan reduces renal damage progression in db/db mice. Am. J. Physiol. Renal Physiol. 292, F321–F329 (2007).
Santiago, B. et al. Topical application of a peptide inhibitor of transforming growth factor-beta1 ameliorates bleomycin-induced skin fibrosis. J. Invest. Dermatol. 125, 450–455 (2005).
Munger, J. S. et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Kaminski, N. et al. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc. Natl Acad. Sci. USA 97, 1778–1783 (2000).
Horan, G. S. et al. Partial inhibition of integrin α(v)β6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 177, 56–65 (2008).
Puthawala, K. et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 177, 82–90 (2008).
Munger, J. S. et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Horan, G. S. et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 177, 56–65 (2008).
Mori, Y. et al. Selective inhibition of activin receptor-like kinase 5 signaling blocks profibrotic transforming growth factor beta responses in skin fibroblasts. Arthritis Rheum. 50, 4008–4021 (2004).
Ishida, W. et al. Intracellular TGF-beta receptor blockade abrogates SMAD-dependent fibroblast activation in vitro and in vivo. J. Invest. Dermatol. 126, 1733–1744 (2006).
Moon, J. A. et al. IN-1130, a novel transforming growth factor-β type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 70, 1234–1243.
de Gouville, A. C. et al. Inhibition of TGF-beta signaling by an TGFBR1 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br. J. Pharmacol. 145, 166–177.
Fu, K. et al. SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler. Thromb. Vasc. Biol. 28, 665–671 (2008).
Bonniaud, P. et al. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active TGFBR1 kinase inhibitor. Am. J. Respir. Crit. Care Med. 171, 889–898.
Petersen, M. et al. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 73, 705–715 (2008).
Itoh, S. & ten Dijke, P. Negative regulation of TGF-beta receptor/SMAD signal transduction. Curr. Opin. Cell Biol. 19, 176–184 (2007).
Nakao, A. et al. Transient gene transfer and expression of SMAD7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest. 104, 5–11 (1999).
Nie, J. et al. SMAD7 gene transfer inhibits peritoneal fibrosis. Kidney Int. 72, 1336–1344 (2007).
Saika, S. et al. Effect of SMAD7 gene overexpression on transforming growth factor beta-induced retinal pigment fibrosis in a proliferative vitreoretinopathy mouse model. Arch. Ophthalmol. 125, 647–654 (2007).
Lan, H. Y. SMAD7 as a therapeutic agent for chronic kidney diseases. Front. Biosci. 13, 4984–4992 (2008).
Shukla, M. N. et al. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via SMAD7. Am. J. Respir. Cell. Mol. Biol. (2008).
Angion Biomedica Corp [http://angion.com/products.asp] (accessed 20 January 2009).
Weng, H. et al. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor SMADs. J. Hepatol. 46, 295–303 (2007).
Liu, X. et al. Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med. 2, e354 (2005).
Zhao, B. M. & Hoffmann, F. M. Inhibition of transforming growth factor-beta1-induced signaling and epithelial-to-mesenchymal transition by the SMAD-binding peptide aptamer Trx-SARA. Mol. Biol. Cell 17, 3819–3831 (2006).
Daniels, C. E. et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J. Clin. Invest. 114, 1308–1316 (2004).
Ishida, W. et al. Novel role of c-Abl tyrosine kinase in profibrotic TGF-Beta responses: selective modulation by the anticancer drug imatinib methylate (gleevec). Arthitis Rheum. 54, S776 (2006).
Bhattacharyya, S. et al. c-Abl mediates profibrotic responses in fibroblasts via Egr-1: inhibition by imatinib. Oncogene (in press).
Chung, L. et al. Molecular framework for response to imatinib mesylate in systemic sclerosis. Arthritis Rheum. (in press).
Krause, D. S. & Van Etten, R. A. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 353, 172–187 (2005).
Distler, J. H. et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 56, 311–322 (2007).
Soria, A. et al. The effect of imatinib (Glivec) on scleroderma and normal dermal fibroblasts: a preclinical study. Dermatology 216, 109–117 (2008).
Pannu, J. et al. SMAD1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 58, 2528–2537 (2008).
Aono, Y. et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 171, 1279–1285 (2005).
Wang, S. et al. Imatinib mesylate blocks a non-SMAD TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 19, 1–11 (2005).
Vittal, R. et al. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J. Pharmacol. Exp. Ther. 321, 35–44 (2007).
van Daele, P. L. et al. Is imatinib mesylate a promising drug in systemic sclerosis? Arthritis Rheum. 58, 2549–2552 (2008).
Sabnani, I. et al. A novel therapeutic approach to the treatment of scleroderma-associated pulmonary complications: safety and efficacy of combination therapy with imatinib and cyclophosphamide. Rheumatology (Oxford) 48, 49–52 (2009).
Sfikakis, P. P. et al. Imatinib for the treatment of refractory, diffuse systemic sclerosis. Rheumatology (Oxford) 47, 735–737 (2008).
Magro, L. et al. Efficacy of imatinib mesylate in the treatment of refractory sclerodermatous chronic GVHD. Bone Marrow Transplant 42, 757–760 (2008).
Moreno-Romero, J. A. et al. Imatinib as a potential treatment for sclerodermatous chronic graft-vs-host disease. Arch. Dermatol. 144, 1106–1109 (2008).
Kay, J. & High, W. A. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum. 58, 2543–2548 (2008).
Azuma, M. et al. Role of alpha1-acid glycoprotein in therapeutic antifibrotic effects of imatinib with macrolides in mice. Am. J. Respir. Crit. Care Med. 176, 1243–1250 (2007).
Kucharz, E. J. et al. Acute-phase proteins in patients with systemic sclerosis. Clin. Rheumatol. 2, 165–166 (2000).
Fietta, A. et al. Analysis of bronchoalveolar lavage fluid proteome from systemic sclerosis patients with or without functional, clinical and radiological signs of lung fibrosis. Arthritis Res. Ther. 8, R160 (2006).
Druker, B. J. et al.; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Schermuly, R. T. et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 115, 2811–2821 (2005).
Perros, F. et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 178, 81–88 (2008).
Patterson, K. C. et al. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann. Intern. Med. 145, 152–153 (2006).
Ghofrani, H. A. et al. Imatinib for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 353, 1412–1413 (2005).
ClinicalTrials.gov [http://clinicaltrials.gov/ct2/show/NCT00764309] (accessed 27 January 2009).
Rosenbloom, J. & Jiménez, S. A. Molecular ablation of transforming growth factor beta signaling pathways by tyrosine kinase inhibition: the coming of a promising new era in the treatment of tissue fibrosis. Arthritis Rheum. 58, 2219–2224 (2008).
Ghosh, A. K. et al. Trichostatin A blocks TGF-beta-induced collagen gene expression in skin fibroblasts: involvement of Sp1. Biochem. Biophys. Res. Commun. 354, 420–426 (2007).
Wang, Y. et al. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 54, 2271–2279 (2006).
Huber, L. C. et al. Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum. 56, 2755–2764 (2007).
Pennison, M. & Pasche, B. Targeting transforming growth factor-beta signaling. Curr. Opin. Oncol. 19, 579–585 (2007).
Ruzek, M. C. et al. Minimal effects on immune parameters following chronic anti-TGF-beta monoclonal antibody administration to normal mice. Immunopharmacol. Immunotoxicol. 25, 235–257 (2003).
Yang, Y. A. et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J. Clin. Invest. 109, 1607–1615 (2002).
Habashi, J. P. et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121 (2006).
Cohn, R. D. et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13, 204–210 (2007).
De Angelis, R. et al. Diffuse scleroderma occurring after the use of paclitaxel for ovarian cancer. Clin. Rheumatol. 22, 49–52 (2003).
Kupfer, I. et al. Scleroderma-like cutaneous lesions induced by paclitaxel: a case study. J. Am. Acad. Dermatol. 48, 279–281 (2003).
Ghosh, A. K. et al. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 50, 1305–1318 (2004).
Sime, P. J. The antifibrogenic potential of PPARgamma ligands in pulmonary fibrosis. J. Investig. Med. 56, 534–538 (2008).
Milam, J. E. et al. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L891–L901 (2008).
Genovese, T. et al. Effect of rosiglitazone and 15-deoxy-Delta12, 14-prostaglandin J2 on bleomycin-induced lung injury. Eur. Respir. J. 25, 225–234 (2005).
Wu, M. et al. Rosiglitazone modulates fibrotic responses in mice via peroxisome proliferators activated receptor gamma: implications for systemic sclerosis. Am. J. Pathol. (in press).
Shi-wen, X. et al. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 56, 4189–4194 (2007).
Silver, R. M. Endothelin and scleroderma lung disease. Rheumatology (Oxford) 47 (Suppl. 5), v25–v26 (2008).
Yamada, H. et al. Tranilast inhibits collagen synthesis in normal, scleroderma and keloid fibroblasts at a late passage culture but not at an early passage culture. J. Dermatol. Sci. 9, 45–47 (1995).
Platten, M. et al. N-[3, 4-dimethoxycinnamoyl]-anthranilic acid (tranilast) inhibits transforming growth factor-beta release and reduces migration and invasiveness of human malignant glioma cells. Int. J. Cancer 93, 53–61 (2001).
Acknowledgements
Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Both authors have received grants/research support from the NIH. J Varga has also received a grant/research support from Novartis.
Rights and permissions
About this article
Cite this article
Varga, J., Pasche, B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 5, 200–206 (2009). https://doi.org/10.1038/nrrheum.2009.26
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.26
This article is cited by
-
Novel insights into systemic sclerosis using a sensitive computational method to analyze whole-genome bisulfite sequencing data
Clinical Epigenetics (2023)
-
Podocarpusflavone alleviated renal fibrosis in obstructive nephropathy by inhibiting Fyn/Stat3 signaling pathway
Journal of Natural Medicines (2023)
-
Understanding and Therapeutically Targeting the Scleroderma Myofibroblast
Current Treatment Options in Rheumatology (2022)
-
Immune Inhibitory Properties and Therapeutic Prospects of Transforming Growth Factor-Beta and Interleukin 10 in Autoimmune Hepatitis
Digestive Diseases and Sciences (2022)
-
Novel role of long non-coding RNAs in autoimmune cutaneous disease
Journal of Cell Communication and Signaling (2022)