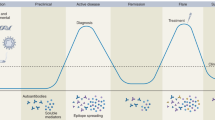
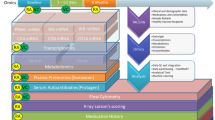
Abstract
Rheumatologists see patients with a range of autoimmune diseases. Phenotyping these diseases for diagnosis, prognosis and selection of therapies is an ever increasing problem. Advances in multiplexed assay technology at the gene, protein, and cellular level have enabled the identification of 'actionable biomarkers'; that is, biological metrics that can inform clinical practice. Not only will such biomarkers yield insight into the development, remission, and exacerbation of a disease, they will undoubtedly improve diagnostic sensitivity and accuracy of classification, and ultimately guide treatment. This Review provides an introduction to these powerful technologies that could promote the identification of actionable biomarkers, including mass cytometry, protein arrays, and immunoglobulin and T-cell receptor high-throughput sequencing. In our opinion, these technologies should become part of routine clinical practice for the management of autoimmune diseases. The use of analytical tools to deconvolve the data obtained from use of these technologies is also presented here. These analyses are revealing a more comprehensive and interconnected view of the immune system than ever before and should have an important role in directing future treatment approaches for autoimmune diseases.
Key Points
-
Antigen arrays are valuable for profiling autoantibodies in diverse rheumatic autoimmune diseases and can be composed of most biomolecules including proteins, peptides, protein complexes, sugars, nucleic acids and lipids
-
High-throughput DNA sequencing enables the tracking of disease-associated clones of T cells and B cells in autoimmune diseases; changes in populations of these cells can be correlated with therapeutic response
-
The analysis of peripheral blood cells following cellular activation might be important in identifying clinically actionable biomarkers
-
New technologies enable analysis of gene and protein expression in whole blood samples; deconvolution of datasets reveals which immune-cell subset underlies a change without isolating or manipulating the cells
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
01 October 2012
In the versions of this article initially published in print and online, the authors did not include acknowledgements of grant support as required by their funding policies. This omission has been corrected for the HTML and PDF versions of the article.
References
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 1, 493–502 (2002).
Nahta, R. & Esteva, F. J. HER-2-targeted therapy: lessons learned and future directions. Clin. Cancer Res. 9, 5078–5084 (2003).
LaGasse, J. M. et al. Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care 25, 505–511 (2002).
van der Woude, D. et al. The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann. Rheum. Dis. 69, 1110–1116 (2010).
Zethelius, B. et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N. Engl. J. Med. 358, 2107–2116 (2008).
Maclaren, N. et al. Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2β predict immune-mediated (Type 1) diabetes in relatives. J. Autoimmun. 12, 279–287 (1999).
Goekoop-Ruiterman, Y. P. et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 52, 3381–3390 (2005).
Moreland, L. W. et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N. Engl. J. Med. 337, 141–147 (1997).
Li, Q. Z. et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin. Exp. Immunol. 147, 60–70 (2007).
Sokolove, J. et al. Autoantibody Epitope Spreading in the Pre-Clinical Phase Predicts Progression to Rheumatoid Arthritis. PLoS One (in press).
Hueber, W. et al. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res. Ther. 11, R76 (2009).
Querec, T. D. et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10, 116–125 (2009).
Irish, J. M. et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118, 217–228 (2004).
Bandura, D. R. et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009).
Ornatsky, O. et al. Highly multiparametric analysis by mass cytometry. J. Immunol. Methods 361, 1–20 (2010).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Edwards, B. S., Oprea, T., Prossnitz, E. R. & Sklar, L. A. Flow cytometry for high-throughput, high-content screening. Curr. Opin. Chem. Biol. 8, 392–398 (2004).
Pyne, S. et al. Automated high-dimensional flow cytometric data analysis. Proc. Natl Acad. Sci. USA 106, 8519–8524 (2009).
Walther, G. et al. Automatic clustering of flow cytometry data with density-based merging. Adv. Bioinformatics 686759 (2009).
Qiu, P. et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol. 29, 886–891 (2011).
Stanford University Gary P. Nolan Laboratory. CytoSPADE: cytoscape-driven spanning tree progression of density-normalised events [online], (2011).
Robinson, W. H. et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 8, 295–301 (2002).
Bussow, K. et al. A method for global protein expression and antibody screening on high-density filters of an arrayed cDNA library. Nucleic Acids Res. 26, 5007–5008 (1998).
Ekins, R. P. Multi-analyte immunoassay. J. Pharm. Biomed. Anal. 7, 155–168 (1989).
Joos, T. O. et al. A microarray enzyme-linked immunosorbent assay for autoimmune diagnostics. Electrophoresis 21, 2641–2650 (2000).
Kanter, J. L. et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 12, 138–143 (2006).
Wang, D., Liu, S., Trummer, B. J., Deng, C. & Wang, A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat. Biotechnol. 20, 275–281 (2002).
Quintana, F. J. et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl Acad. Sci. USA 105, 18889–18894 (2008).
Michaud, G. A. et al. Analyzing antibody specificity with whole proteome microarrays. Nat. Biotechnol. 21, 1509–1512 (2003).
Zhu, H. et al. Global analysis of protein activities using proteome chips. Science 293, 2101–2105 (2001).
Tabakman, S. M. et al. Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat. Commun. 2, 466 (2011).
Chen, R. J. et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl Acad. Sci. USA 100, 4984–4989 (2003).
Chen, Z. et al. Protein microarrays with carbon nanotubes as multicolor Raman labels. Nat. Biotechnol. 26, 1285–1292 (2008).
Gaster, R. S. et al. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol. 6, 314–320 (2011).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Garren, H. et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann. Neurol. 63, 611–620 (2008).
Robinson, W. H. et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 (2003).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
von Muhlen, C. A. & Tan, E. M. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin. Arthritis Rheum. 24, 323–358 (1995).
Campbell, P. J. et al. Subclonal phylogenetic structures in cancer revealed by ultra-deep sequencing. Proc. Natl Acad. Sci. USA 105, 13081–13086 (2008).
Liu, F., Whitton, J. L. & Slifka, M. K. The rapidity with which virus-specific CD8+ T cells initiate IFN-gamma synthesis increases markedly over the course of infection and correlates with immunodominance. J. Immunol. 173, 456–462 (2004).
Meffre, E. et al. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Invest. 108, 879–886 (2001).
Weinstein, J. A., Jiang, N., White, R. A. 3rd, Fisher, D. S. & Quake, S. R. High-throughput sequencing of the zebrafish antibody repertoire. Science 324, 807–810 (2009).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Crow, M. K., Kirou, K. A. & Wohlgemuth, J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity 36, 481–490 (2003).
Lau, C. M. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 (2005).
Rifkin, I. R., Leadbetter, E. A., Busconi, L., Viglianti, G. & Marshak-Rothstein, A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol. Rev. 204, 27–42 (2005).
Bauer, J. W. et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 3, e491 (2006).
Bauer, J. W. et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 60, 3098–3107 (2009).
Hueber, W. et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann. Rheum. Dis. 66, 712–719 (2007).
Axtell, R. C. et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 16, 406–412 (2010).
Green, N. M. & Marshak-Rothstein, A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin. Immunol. 23, 106–112 (2011).
Sharp, V. & Utz, P. J. Technology insight: can autoantibody profiling improve clinical practice? Nat. Clin. Pract. Rheumatol. 3, 96–103 (2007).
Holst, J. et al. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat. Immunol. 9, 658–666 (2008)
Richez, C. et al. IFN regulatory factor 5 is required for disease development in the FcγRIIB−/−Yaa and FcγRIIB−/− mouse models of systemic lupus erythematosus. J. Immunol. 184, 796–806 (2010).
Thibault, D. L. et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J. Clin. Invest. 118, 1417–1426 (2008).
Thibault, D. L. et al. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis Res. Ther. 11, R112 (2009).
Kattah, M. G., Coller, J., Cheung, R. K., Oshidary, N. & Utz, P. J. HIT: a versatile proteomics platform for multianalyte phenotyping of cytokines, intracellular proteins and surface molecules. Nat. Med. 14, 1284–1289 (2008).
Price, J. “On silico” peptide microarrays for high resolution mapping of antibody epitopes and diverse protein-protein interactions. Nat. Med. (in press).
Bentley, D. R. et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456, 53–59 (2008).
Boyd, S. D. et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci. Transl. Med. 1, 12ra23 (2009).
Freeman, J. D., Warren, R. L., Webb, J. R., Nelson, B. H. & Holt, R. A. Profiling the T-cell receptor β-chain repertoire by massively parallel sequencing. Genome Res. 19, 1817–1824 (2009).
Glanville, J. et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc. Natl Acad. Sci. USA 106, 20216–20221 (2009).
Margulies, M. et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380 (2005).
Robins, H. S. et al. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood 114, 4099–4107 (2009).
Venturi, V. et al. A mechanism for TCR sharing between T cell subsets and individuals revealed by pyrosequencing. J. Immunol. 186, 4285–4294 (2011).
Wang, C. et al. High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc. Natl Acad. Sci. USA 107, 1518–1523 (2010).
Wu, Y. C. et al. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood 116, 1070–1078 (2010).
Liao, H. X. et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J. Exp. Med. 208, 2237–2249 (2011).
Wu, X. et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333, 1593–1602 (2011).
Robins, H. et al. Ultra-sensitive detection of rare T cell clones. J. Immunol. Methods 375, 14–19 (2011).
Warren, R. L. et al. Exhaustive T-cell repertoire sequencing of human peripheral blood samples reveals signatures of antigen selection and a directly measured repertoire size of at least 1 million clonotypes. Genome Res. 21, 790–797 (2011).
Lebecque, S. G. & Gearhart, P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J. Exp. Med. 172, 1717–1727 (1990).
Boyd, S. D. et al. Individual variation in the germline Ig gene repertoire inferred from variable region gene rearrangements. J. Immunol. 184, 6986–6992 (2010).
Wang, Y. et al. Genomic screening by 454 pyrosequencing identifies a new human IGHV gene and sixteen other new IGHV allelic variants. Immunogenetics 63, 259–265 (2011).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Yurasov, S. et al. Persistent expression of autoantibodies in SLE patients in remission. J. Exp. Med. 203, 2255–2261 (2006).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005).
Edwards, B. M. et al. The remarkable flexibility of the human antibody repertoire; isolation of over one thousand different antibodies to a single protein, BLyS. J. Mol. Biol. 334, 103–118 (2003).
Glanville, J. et al. Naive antibody gene-segment frequencies are heritable and unaltered by chronic lymphocyte ablation. Proc. Natl Acad. Sci. USA 108, 20066–20071 (2011).
Jackson, K. J. et al. Divergent human populations show extensive shared IGK rearrangements in peripheral blood B cells. Immunogenetics 64, 3–14 (2011).
Massengill, S. F., Goodenow, M. M. & Sleasman, J. W. SLE nephritis is associated with an oligoclonal expansion of intrarenal T cells. Am. J. Kidney Dis. 31, 418–426 (1998).
Murata, H. et al. T cell receptor repertoire of T cells in the kidneys of patients with lupus nephritis. Arthritis Rheum. 46, 2141–2147 (2002).
Winchester, R. et al. Immunologic characteristics of intrarenal T cells: Trafficking of expanded CD8 T cell β-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. http://dx.doi.org/10.1002/art.33488.
Gillespie, G. M. et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 74, 8140–8150 (2000).
Hadrup, S. R. et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176, 2645–2653 (2006).
Janeway, C. et al. Immunobiology: The Immune System in Health and Disease (5th edition) (Garland Science, New York, 2001).
Cobb, J. P. et al. Application of genome-wide expression analysis to human health and disease. Proc. Natl Acad. Sci. USA 102, 4801–4806 (2005).
Whitney, A. R. et al. Individuality and variation in gene expression patterns in human blood. Proc. Natl Acad. Sci. USA 100, 1896–1901 (2003).
Xu, Q. et al. Investigation of variation in gene expression profiling of human blood by extended principle component analysis. PLoS ONE 6, e26905 (2011).
Palmer, C., Diehn, M., Alizadeh, A. A. & Brown, P. O. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics 7, 115 (2006).
Clarke, J., Seo, P. & Clarke, B. Statistical expression deconvolution from mixed tissue samples. Bioinformatics 26, 1043–1049 (2010).
Kuroda, M. J. et al. Human immunodeficiency virus type 1 envelope epitope-specific CD4(+) T lymphocytes in simian/human immunodeficiency virus-infected and vaccinated rhesus monkeys detected using a peptide-major histocompatibility complex class II tetramer. J. Virol. 74, 8751–8756 (2000).
Shen-Orr, S. S. et al. Cell type-specific gene expression differences in complex tissues. Nat. Methods 7, 287–289 (2010).
van Lochem, E. G. et al. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry B. Clin. Cytom. 60, 1–13 (2004).
Acknowledgements
The authors wish to thank Dr Hongwu Du for his advice on the systems immunology section of the article and for assistance with editing the manuscript. In addition, the authors thank members of the Boyd lab and Dr A. Fire (Stanford University, Stanford, USA) in collaboration with Dr A. Lucas and Dr L. Liu (Children's Hospital Oakland Research Institute, Oakland, USA) for providing the basis for Figure 4. Furthermore, the authors gratefully acknowledge sources of funding for their research activities: H. T. Maecker's work was supported by NIH grants 3 U19 AI057229, 4 U19 AI090019, and 1 RC4 AG039014; P. J. Utz was the recipient of a Donald E. and Delia B. Baxter Foundation Career Development Award and his work is supported by National Heart, Lung, and Blood Institute (NHLBI) Proteomics contract HHSN288201000034C, Proteomics of Inflammatory Immunity and Pulmonary Arterial Hypertension, NIH grants 5 U19-AI082719, 5 U19-AI050864, 5 U19-AI056363, 1 U19 AI090019 and 4 U19 AI090019, Canadian Institutes of Health Research grant 2 OR-92141, Alliance for Lupus Research grant number 21858, a gift from the Ben May Trust and a gift from the Floren Family Trust, and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 261382; S. D. Boyd is the recipient of NIH grants U19AI090019, U19AI067854, U54-AI065359, UM1AI100663-01, Ellison Medical Foundation grant AG-NS-792-11, and the Lucille Packard Pediatric Research Fund; the work of S. S. Shen-Orr is supported by NIH grant 3 U19 AI057229.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, substantially contributed to the discussion of content and selection of references and reviewed/edited the manuscript before submission. H. T. Maecker, T. M. Lindstrom, W. H. Robinson, P. J. Utz, M. Hale, S. D. Boyd and S. S. Shen-Orr wrote the article.
Corresponding author
Ethics declarations
Competing interests
S. D. Boyd is a consultant for ImmuMetrix LLC, and has received consultancy fees and equity compensation. The remaining authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Maecker, H., Lindstrom, T., Robinson, W. et al. New tools for classification and monitoring of autoimmune diseases. Nat Rev Rheumatol 8, 317–328 (2012). https://doi.org/10.1038/nrrheum.2012.66
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.66