Abstract
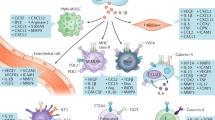
The tolerance state that exists between renal cell carcinoma (RCC) and the host's immune system would be an ideal situation in the setting of human kidney transplantation, in which graft tolerance is the ultimate goal of immunosuppressive therapy. On the other hand, acute rejection, as it appears in renal allografts, would be the optimal immunologic situation in patients with RCC. Analysis of the underlying mechanisms of acute allograft rejection and local pro-tumor immunosuppression could help to identify potential therapeutic targets for inducing immune tolerance in allograft recipients and immune rejection in RCC patients. Experimental kidney transplantation might be a suitable model in which to analyze these processes. Macrophages are a prominent and vital cell type in the cellular infiltrate seen in both RCC and renal allografts. Depending on their polarization, they can initiate and promote either proinflammatory or pro-tumor responses, which lead to tissue rejection or acceptance, respectively. Improved understanding of macrophage biology could lead to therapeutic modification of their function in order to promote a desirable immunologic response in either RCC or transplant tissue.
Key Points
-
Renal cell carcinoma (RCC) is an immunoresponsive tumor, for which emerging, immune-based treatments are being tested in clinical studies
-
The immunosuppressive tumor microenvironment inhibits an effective immune response by inducing functional anergy of local macrophages and T cells
-
Acute renal allograft rejection is a useful model in which to study the inflammatory and immune mechanisms required to induce tumor rejection
-
Macrophages show a functional plasticity, displaying either an M1 (proinflammatory) or M2 (immunosuppressive and pro-tumor) phenotype
-
Potential therapeutic strategies that target macrophages include the induction of macrophage depletion or reprogramming, and interference with macrophage-mediated co-stimulation of T cells
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ljungberg, B. et al. Renal cell carcinoma guideline. Eur. Urol. 51, 1502–1510 (2007).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
McDermott, D. F. The application of high-dose interleukin-2 for metastatic renal cell carcinoma. Med. Oncol. 26 (Suppl. 1), 13–17 (2009).
The high-dose aldesleukin (IL-2) “Select” trial for patients with metastatic renal cell carcinoma (SELECT) [online].
Van Poppel, H., Joniau, S. & Van Gool, S. W. Vaccine therapy in patients with renal cell carcinoma. Eur. Urol. 55, 1333–1342 (2009).
Rammensee, H. G. Immunology: protein surgery. Nature 427, 203–204 (2004).
van der Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991).
Dengjel, J. et al. Unexpected abundance of HLA class II presented peptides in primary renal cell carcinomas. Clin. Cancer Res. 12, 4163–4170 (2006).
Rammensee, H. G., Weinschenk, T., Gouttefangeas, C. & Stevanovic, S. Towards patient-specific tumor antigen selection for vaccination. Immunol. Rev. 188, 164–176 (2002).
Brossart, P. et al. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 58, 732–736 (1998).
Gouttefangeas, C., Stenzl, A., Stevanovic, S. & Rammensee, H. G. Immunotherapy of renal cell carcinoma. Cancer Immunol. Immunother. 56, 117–128 (2007).
Feyerabend, S. et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 69, 917–927 (2009).
Childs, R. et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N. Engl. J. Med. 343, 750–758 (2000).
Fishman, M. et al. Phase II trial of B7–1 (CD-86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin-2 for treatment of stage IV renal cell carcinoma. J. Immunother. 31, 72–80 (2008).
Jocham, D. et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 363, 594–599 (2004).
May, M. et al. Ten-year survival analysis for renal carcinoma patients treated with an autologous tumour lysate vaccine in an adjuvant setting. Cancer Immunol. Immunother. 59, 687–695 (2010).
Wood, C. et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 372, 145–154 (2008).
Bedke, J. et al. Anti-inflammatory effects of αv integrin antagonism in acute kidney allograft rejection. Am. J. Pathol. 171, 1127–1139 (2007).
Bedke, J. et al. Beneficial effects of CCR1 blockade on the progression of chronic renal allograft damage. Am. J. Transplant. 7, 527–537 (2007).
Schreiber, T. H. & Podack, E. R. A critical analysis of the tumour immunosurveillance controversy for 3-MCA-induced sarcomas. Br. J. Cancer 101, 381–386 (2009).
Burnet, M. Cancer; a biological approach. I. The processes of control. Br. Med. J. 1, 779–786 (1957).
Kasiske, B. L., Snyder, J. J., Gilbertson, D. T. & Wang, C. Cancer after kidney transplantation in the United States. Am. J. Transplant. 4, 905–913 (2004).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Fisher, M. S. & Kripke, M. L. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc. Natl Acad. Sci. USA 74, 1688–1692 (1977).
Schwarz, T. 25 years of UV-induced immunosuppression mediated by T cells—from disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol. 84, 10–18 (2008).
Koebel, C. M. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007).
Kondo, T. et al. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci. 97, 780–786 (2006).
Curiel, T. J. Tregs and rethinking cancer immunotherapy. J. Clin. Invest. 117, 1167–1174 (2007).
Kusmartsev, S. & Vieweg, J. Enhancing the efficacy of cancer vaccines in urologic oncology: new directions. Nat. Rev. Urol. 6, 540–549 (2009).
Kusmartsev, S. et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 14, 8270–8278 (2008).
Collison, L. W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Curiel, T. J. Regulatory T cells and treatment of cancer. Curr. Opin. Immunol. 20, 241–246 (2008).
Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115, 3623–3633 (2005).
Li, J. F. et al. The prognostic value of peritumoral regulatory T cells and its correlation with intratumoral cyclooxygenase-2 expression in clear cell renal cell carcinoma. BJU Int. 103, 399–405 (2009).
Ko, J. S. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 (2009).
Zhao, W., Gu, Y. H., Song, R., Qu, B. Q. & Xu, Q. Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia 22, 1226–1233 (2008).
Lob, S., Konigsrainer, A., Rammensee, H. G., Opelz, G. & Terness, P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat. Rev. Cancer 9, 445–452 (2009).
Lob, S. et al. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111, 2152–2154 (2008).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004).
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Semenza, G. L. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 39, 231–234 (2007).
Gunther, E. & Walter, L. The major histocompatibility complex of the rat (Rattus norvegicus). Immunogenetics 53, 520–542 (2001).
Grone, H. J. et al. Met–RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 13, 1371–1383 (1999).
Hojo, M. et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 397, 530–534 (1999).
Koehl, G. E. et al. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation 77, 1319–1326 (2004).
Maluccio, M. et al. Tacrolimus enhances transforming growth factor-β1 expression and promotes tumor progression. Transplantation 76, 597–602 (2003).
Coffelt, S. B., Hughes, R. & Lewis, C. E. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta 1796, 11–18 (2009).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
de Visser, K. E., Eichten, A. & Coussens, L. M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 6, 24–37 (2006).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Siciliano, N. A., Skinner, J. A. & Yuk, M. H. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J. Immunol. 177, 7131–7138 (2006).
Guiducci, C., Vicari, A. P., Sangaletti, S., Trinchieri, G. & Colombo, M. P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 65, 3437–3446 (2005).
Ricardo, S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
Cua, D. J. & Stohlman, S. A. In vivo effects of T helper cell type 2 cytokines on macrophage antigen-presenting cell induction of T helper subsets. J. Immunol. 159, 5834–5840 (1997).
Savage, N. D. et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFβ-1. J. Immunol. 181, 2220–2226 (2008).
Biswas, S. K. et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation). Blood 107, 2112–2122 (2006).
Sica, A. & Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 117, 1155–1166 (2007).
Nelson, P. J. & Krensky, A. M. Chemokines, chemokine receptors, and allograft rejection. Immunity 14, 377–386 (2001).
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Furuichi, K., Kaneko, S. & Wada, T. Chemokine/chemokine receptor-mediated inflammation regulates pathologic changes from acute kidney injury to chronic kidney disease. Clin. Exp. Nephrol. 13, 9–14 (2009).
Bedke, J. et al. A novel CXCL8 protein-based antagonist in acute experimental renal allograft damage. Mol. Immunol. 47, 1047–1057 (2010).
Stojanovic, T. et al. Met–RANTES inhibition of mucosal perfusion failure in acute intestinal transplant rejection—role of endothelial cell-leukocyte interaction. J. Vasc. Res. 39, 51–58 (2002).
Fischereder, M. et al. CC chemokine receptor 5 and renal-transplant survival. Lancet 357, 1758–1761 (2001).
Robinson, S. C. et al. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 63, 8360–8365 (2003).
Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 (2004).
Kondo, T. et al. High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. J. Urol. 171, 2171–2175 (2004).
Roca, H. et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem. 284, 34342–34354 (2009).
Jose, M. D., Ikezumi, Y., van Rooijen, N., Atkins, R. C. & Chadban, S. J. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation 76, 1015–1022 (2003).
Soltau, J. et al. Antitumoral and antiangiogenic efficacy of bisphosphonates in vitro and in a murine RENCA model. Anticancer Res. 28, 933–941 (2008).
Miwa, S. et al. A case of bone, lung, pleural and liver metastases from renal cell carcinoma which responded remarkably well to zoledronic acid monotherapy. Jpn J. Clin. Oncol. 39, 745–750 (2009).
Barleon, B. et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87, 3336–3343 (1996).
Salnikov, A. V. et al. Inhibition of carcinoma cell-derived VEGF reduces inflammatory characteristics in xenograft carcinoma. Int. J. Cancer 119, 2795–2802 (2006).
Roland, C. L. et al. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol. Cancer Ther. 8, 1761–1771 (2009).
Kreitman, R. J. Recombinant immunotoxins for the treatment of chemoresistant hematologic malignancies. Curr. Pharm. Des. 15, 2652–2664 (2009).
Foss, F. Clinical experience with denileukin diftitox (ONTAK). Semin. Oncol. 33 (1 Suppl. 3), S11–S16 (2006).
Denileukin diftitox and interleukin-2 in treating patients with metastatic kidney cancer [online].
Stout, R. D., Watkins, S. K. & Suttles, J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J. Leukoc. Biol. 86, 1105–1109 (2009).
Hill, H. C. et al. Cancer immunotherapy with interleukin 12 and granulocyte-macrophage colony-stimulating factor-encapsulated microspheres: coinduction of innate and adaptive antitumor immunity and cure of disseminated disease. Cancer Res. 62, 7254–7263 (2002).
Watkins, S. K., Egilmez, N. K., Suttles, J. & Stout, R. D. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J. Immunol. 178, 1357–1362 (2007).
Brunda, M. J. et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 178, 1223–1230 (1993).
Coughlin, C. M. et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J. Clin. Invest. 101, 1441–1452 (1998).
Hwang, K. S. et al. Adenovirus-mediated interleukin-12 gene transfer combined with cytosine deaminase followed by 5-fluorocytosine treatment exerts potent antitumor activity in Renca tumor-bearing mice. BMC Cancer 5, 51 (2005).
Schaefer, L. et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 115, 2223–2233 (2005).
Dehmel, S. et al. Chemokine receptor CCR5 deficiency induces alternative macrophage activation and improves long-term renal allograft outcome. Eur. J. Immunol. 40, 267–278 (2010).
Dougan, M. & Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 27, 83–117 (2009).
Sayegh, M. H. & Turka, L. A. The role of T-cell costimulatory activation pathways in transplant rejection. N. Engl. J. Med. 338, 1813–1821 (1998).
O'Day, S. J., Hamid, O. & Urba, W. J. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer 110, 2614–2627 (2007).
Salomon, B. & Bluestone, J. A. Complexities of CD28/B7:CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19, 225–252 (2001).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Shiao, S. L. et al. Immunomodulatory properties of FK734, a humanized anti-CD28 monoclonal antibody with agonistic and antagonistic activities. Transplantation 83, 304–313 (2007).
Larsen, C. P. et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4–Ig with potent immunosuppressive properties. Am. J. Transplant. 5, 443–453 (2005).
Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Yang, J. C. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 30, 825–830 (2007).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Acknowledgements
A. Stenzl was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, SFB 685 C5). The authors thank Hermann-Josef Gröne, Department of Cellular and Molecular Pathology, German Cancer Research Center, Heidelberg, Germany, and Hans-Georg Rammensee, Department of Immunology, University of Tübingen, Germany for their careful reading of this manuscript and for their thoughtful comments. The authors additionally thank Hermann-Josef Gröne for providing the photomicrographs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bedke, J., Stenzl, A. Immunologic mechanisms in RCC and allogeneic renal transplant rejection. Nat Rev Urol 7, 339–347 (2010). https://doi.org/10.1038/nrurol.2010.59
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2010.59
This article is cited by
-
Dramatic early event in chronic allograft nephropathy: increased but not decreased expression of MMP-9 gene
Diagnostic Pathology (2013)
-
Prostatakarzinom
Der Urologe (2012)
-
Nierenzellkarzinom
Der Urologe (2010)