Abstract
Complete testicular descent is a sign of, and a prerequisite for, normal testicular function in adult life. The process of testis descent is dependent on gubernacular growth and reorganization, which is regulated by the Leydig cell hormones insulin-like peptide 3 (INSL3) and testosterone. Investigation of the role of INSL3 and its receptor, relaxin-family peptide receptor 2 (RXFP2), has contributed substantially to our understanding of the hormonal control of testicular descent. Cryptorchidism is a common congenital malformation, which is seen in 2–9% of newborn boys, and confers an increased risk of infertility and testicular cancer in adulthood. Although some cases of isolated cryptorchidism in humans can be ascribed to known genetic defects, such as mutations in INSL3 or RXFP2, the cause of cryptorchidism remains unknown in most patients. Several animal and human studies are currently underway to test the hypothesis that in utero factors, including environmental and maternal lifestyle factors, may be involved in the etiology of cryptorchidism. Overall, the etiology of isolated cryptorchidism seems to be complex and multifactorial, involving both genetic and nongenetic components.
Key Points
-
The process of testicular descent occurs during fetal development and should be completed before birth in humans
-
Undescended testes, or cryptorchidism, confers an increased risk of testicular cancer and infertility in adult life
-
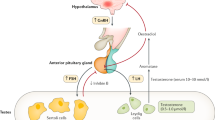
Testosterone and insulin-like peptide 3 produced by the Leydig cells both have a pivotal role in testicular descent
-
Testicular descent relies primarily on hormone-dependent development and reorganization of the ligament gubernaculum
-
The intrauterine environment is often involved in the pathogenesis of isolated cryptorchidism, whereas known genetic factors are responsible for only a small proportion of cases
-
The etiology of cryptorchidism is incompletely understood, and is regarded as both complex and multifactorial
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lee, P. A. & Coughlin, M. T. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm. Res. 55, 28–32 (2001).
Cortes, D., Thorup, J. M. & Visfeldt, J. Cryptorchidism: aspects of fertility and neoplasms. A study including data of 1,335 consecutive boys who underwent testicular biopsy simultaneously with surgery for cryptorchidism. Horm. Res. 55, 21–27 (2001).
Dieckmann, K. P. & Pichlmeier, U. Clinical epidemiology of testicular germ cell tumors. World J. Urol. 22, 2–14 (2004).
Schnack, T. H., Poulsen, G., Myrup, C., Wohlfahrt, J. & Melbye, M. Familial coaggregation of cryptorchidism, hypospadias, and testicular germ cell cancer: a nationwide cohort study. J. Natl. Cancer Inst. 102, 187–192 (2010).
Brennan, J. & Capel, B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet. 5, 509–521 (2004).
Stukenborg, J. B., Colon, E. & Soder, O. Ontogenesis of testis development and function in humans. Sex Dev. 4, 199–212 (2010).
Geissler, W. M. et al. Male pseudohermaphroditism caused by mutations of testicular 17 beta-hydroxysteroid dehydrogenase 3. Nat. Genet. 7, 34–39 (1994).
Hughes, I. A., Houk, C., Ahmed, S. F. & Lee, P. A. Consensus statement on management of intersex disorders. J. Pediatr. Urol. 2, 148–162 (2006).
Codesal, J., Regadera, J., Nistal, M., Regadera-Sejas, J. & Paniagua, R. Involution of human fetal Leydig cells. An immunohistochemical, ultrastructural and quantitative study. J. Anat. 172, 103–114 (1990).
O'Shaughnessy, P. J. et al. Developmental changes in human fetal testicular cell numbers and messenger ribonucleic acid levels during the second trimester. J. Clin. Endocrinol. Metab. 92, 792–794 (2007).
Svechnikov, K. et al. Origin, development and regulation of human Leydig cells. Horm. Res. Pediatr. 73, 93–101 (2010).
Tapanainen, J., Kellokumpu-Lehtinen, P., Pelliniemi, L. & Huhtaniemi, I. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J. Clin. Endocrinol. Metab. 52, 98–102 (1981).
Lambrot, R. et al. Use of organ culture to study the human fetal testis development: effect of retinoic acid. J. Clin. Endocrinol. Metab. 91, 2696–2703 (2006).
Rabinovici, J. & Jaffe, R. B. Development and regulation of growth and differentiated function in human and subhuman primate fetal gonads. Endocr. Rev. 11, 532–557 (1990).
Sultan, C. et al. Disorders linked to insufficient androgen action in male children. Hum. Reprod. Update 7, 314–322 (2001).
Kremer, H. et al. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat. Genet. 9, 160–164 (1995).
Andersson, A. M. et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J. Clin. Endocrinol. Metab. 83, 675–681 (1998).
Main, K. M., Schmidt, I. M. & Skakkebaek, N. E. A possible role for reproductive hormones in newborn boys: progressive hypogonadism without the postnatal testosterone peak. J. Clin. Endocrinol. Metab. 85, 905–907 (2000).
Hutson, J. M. A biphasic model for the hormonal control of testicular descent. Lancet 2, 419–421 (1985).
Heyns, C. F. The gubernaculum during testicular descent in the human fetus. J. Anat. 153, 93–112 (1987).
Barteczko, K. J. & Jacob, M. I. The testicular descent in human. Origin, development and fate of the gubernaculum Hunteri, processus vaginalis peritonei, and gonadal ligaments. Adv. Anat. Embryol. Cell Biol. 156, 1–98 (2000).
Backhouse, K. M. The gubernaculum testis Hunteri: Testicular descent and maldescent. Arris and Gale lecture delivered at The Royal College of Surgeons of England on 27th October 1959. Ann. R. Coll. Surg. Engl. 35, 15–33 (1964).
Heyns, C. F., Human, H. J., Werely, C. J. & De Klerk, D. P. The glycosaminoglycans of the gubernaculum during testicular descent in the fetus. J. Urol. 143, 612–617 (1990).
Sampaio, F. J. & Favorito, L. A. Analysis of testicular migration during the fetal period in humans. J. Urol. 159, 540–542 (1998).
Costa, W. S., Sampaio, F. J., Favorito, L. A. & Cardoso, L. E. Testicular migration: remodeling of connective tissue and muscle cells in human gubernaculum testis. J. Urol. 167, 2171–2176 (2002).
Huynh, J., Shenker, N. S., Nightingale, S. & Hutson, J. M. Signaling molecules: clues from development of the limb bud for cryptorchidism? Pediatr. Surg. Int. 23, 617–624 (2007).
Rotondi, M. et al. Prenatal measurement of testicular diameter by ultrasonography: development of fetal male gender and evaluation of testicular descent. Prenat. Diagn. 21, 112–115 (2001).
Zimmermann, S. et al. Targeted disruption of the INSL3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 13, 681–691 (1999).
Nef, S. & Parada, L. F. Cryptorchidism in mice mutant for INSL3. Nat. Genet. 22, 295–299 (1999).
Overbeek, P. A. et al. A transgenic insertion causing cryptorchidism in mice. Genesis 30, 26–35 (2001).
Kumagai, J. et al. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J. Biol. Chem. 277, 31283–31286 (2002).
Kubota, Y. et al. The role of insulin 3, testosterone, Müllerian inhibiting substance and relaxin in rat gubernacular growth. Mol. Hum. Reprod. 8, 900–905 (2002).
Kaftanovskaya, E. M. et al. Suppression of Insulin-like3 receptor reveals the role of beta-catenin and Notch signaling in gubernaculum development. Mol. Endocrinol. 25, 170–183 (2011).
Johnson, K. J. et al. Insulin-like 3 exposure of the fetal rat gubernaculum modulates expression of genes involved in neural pathways. Biol. Reprod. 83, 774–782 (2010).
Tomiyama, H., Hutson, J. M., Truong, A. & Agoulnik, A. I. Transabdominal testicular descent is disrupted in mice with deletion of insulinlike factor 3 receptor. J. Pediatr. Surg. 38, 1793–1798 (2003).
Adham, I. M. et al. The overexpression of the insl3 in female mice causes descent of the ovaries. Mol. Endocrinol. 16, 244–252 (2002).
Anand-Ivell, R., Ivell, R., Driscoll, D. & Manson, J. Insulin-like factor 3 levels in amniotic fluid of human male fetuses. Hum. Reprod. 23, 1180–1186 (2008).
Bay, K. et al. Insulin-like factor 3 levels in second-trimester amniotic fluid. J. Clin. Endocrinol. Metab. 93, 4048–4051 (2008).
Josso, N., Belville, C., di, C. N. & Picard, J. Y. AMH and AMH receptor defects in persistent Müllerian duct syndrome. Hum. Reprod. Update 11, 351–356 (2005).
Bartlett, J. E. et al. Gubernacular development in Mullerian inhibiting substance receptor-deficient mice. BJU Int. 89, 113–118 (2002).
Quigley, C. A. et al. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr. Rev. 16, 271–321 (1995).
Ahmed, S. F. et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J. Clin. Endocrinol. Metab. 85, 658–665 (2000).
Hannema, S. E. et al. Testicular development in the complete androgen insensitivity syndrome. J. Pathol. 208, 518–527 (2006).
Hutson, J. M., Baker, M., Terada, M., Zhou, B. & Paxton, G. Hormonal control of testicular descent and the cause of cryptorchidism. Reprod. Fertil. Dev. 6, 151–156 (1994).
Shono, T. et al. Scanning electron microscopy shows inhibited gubernacular development in relation to undescended testes in estrogen-treated mice. Int. J. Androl. 19, 263–270 (1996).
Hosie, S., Wessel, L. & Waag, K. L. Could testicular descent in humans be promoted by direct androgen stimulation of the gubernaculum testis? Eur. J. Pediatr. Surg. 9, 37–41 (1999).
Barthold, J. S., Kumasi-Rivers, K., Upadhyay, J., Shekarriz, B. & Imperato-McGinley, J. Testicular position in the androgen insensitivity syndrome: implications for the role of androgens in testicular descent. J. Urol. 164, 497–501 (2000).
Yong, E. X. et al. Calcitonin gene-related peptide stimulates mitosis in the tip of the rat gubernaculum in vitro and provides the chemotactic signals to control gubernacular migration during testicular descent. J. Pediatr. Surg. 43, 1533–1539 (2008).
Zuccarello, D. et al. Preliminary data suggest that mutations in the CGRP pathway are not involved in human sporadic cryptorchidism. J. Endocrinol. Invest. 27, 760–764 (2004).
Yuan, F. P. et al. The role of RXFP2 in mediating androgen-induced inguinoscrotal testis descent in LH-receptor-knockout mice. Reproduction 139, 759–769 (2010).
Lague, E. & Tremblay, J. J. Antagonistic effects of testosterone and the endocrine disruptor mono-(2-ethylhexyl) phthalate on INSL3 transcription in Leydig cells. Endocrinology 149, 688–694 (2008).
Bay, K. et al. Insulin-like factor 3 levels in cord blood and serum from children: effects of age, postnatal hypothalamic-pituitary-gonadal axis activation, and cryptorchidism. J. Clin. Endocrinol. Metab. 92, 4020–4027 (2007).
Bay, K. et al. Insulin-Like Factor 3 (INSL3) Serum Levels in 135 normal men and 85 men with testicular disorders: relationship to the LH–testosterone axis. J. Clin. Endocrinol. Metab. 90, 3410–3418 (2005).
Anand-Ivell, R. et al. Peripheral INSL3 concentrations decline with age in a large population of Australian men. Int. J. Androl. 29, 618–626 (2006).
Wikstrom, A. M., Bay, K., Hero, M., Andersson, A. M. & Dunkel, L. Serum insulin-like factor 3 levels during puberty in healthy boys and boys with Klinefelter syndrome. J. Clin. Endocrinol. Metab. 91, 4705–4708 (2006).
Bay, K. & Andersson, A. M. Human testicular insulin-like factor 3: in relation to development, reproductive hormones and andrological disorders. Int. J. Androl. doi:10.1111/j.1365–26052010.01074.x.
Ivell, R. et al. Relaxin-like factor: a highly specific and constitutive new marker for Leydig cells in the human testis. Mol. Hum. Reprod. 3, 459–466 (1997).
Kawamura, K. et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc. Natl Acad. Sci. USA 101, 7323–7328 (2004).
Anand-Ivell, R. J. et al. Expression of the insulin-like peptide 3 (INSL3) hormone-receptor (LGR8) system in the testis. Biol. Reprod. 74, 945–953 (2006).
Feng, S. et al. Developmental expression and gene regulation of insulin-like 3 receptor RXFP2 in mouse male reproductive organs. Biol. Reprod. 77, 671–680 (2007).
Nguyen, M. T. et al. Effects of orchiopexy on congenitally cryptorchid insulin-3 knockout mice. J. Urol. 168, 1779–1783 (2002).
Ferlin, A. et al. Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. J. Bone Miner. Res. 23, 683–693 (2008).
Hombach-Klonisch, S. et al. INSL3 has tumor-promoting activity in thyroid cancer. Int. J. Cancer 127, 521–531 (2010).
Scorer, C. G. The descent of the testis. Arch. Dis. Child. 39, 605–609 (1964).
Berkowitz, G. S. et al. Prevalence and natural history of cryptorchidism. Pediatrics 92, 44–49 (1993).
Ghirri, P. et al. Incidence at birth and natural history of cryptorchidism: a study of 10,730 consecutive male infants. J. Endocrinol. Invest. 25, 709–715 (2002).
Boisen, K. A. et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet 363, 1264–1269 (2004).
Preiksa, R. T. et al. Higher than expected prevalence of congenital cryptorchidism in Lithuania: a study of 1204 boys at birth and 1 year follow-up. Hum. Reprod. 20, 1928–1932 (2005).
Acerini, C. L., Miles, H. L., Dunger, D. B., Ong, K. K. & Hughes, I. A. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Arch. Dis. Child. 94, 868–872 (2009).
Toledano, M. B. et al. Temporal trends in orchidopexy, Great Britain, 1992–1998. Environ. Health Perspect. 111, 129–132 (2003).
Capello, S. A., Giorgi, L. J. Jr & Kogan, B. A. Orchiopexy practice patterns in New York State from 1984 to 2002. J. Urol. 176, 1180–1183 (2006).
Cortes, D., Kjellberg, E. M., Breddam, M. & Thorup, J. The true incidence of cryptorchidism in Denmark. J. Urol. 179, 314–318 (2008).
Paulozzi, L. J. International trends in rates of hypospadias and cryptorchidism. Environ. Health Perspect. 107, 297–302 (1999).
Chilvers, C., Pike, M. C., Forman, D., Fogelman, K. & Wadsworth, M. E. Apparent doubling of frequency of undescended testis in England and Wales in 1962–81. Lancet 2, 330–332 (1984).
Hack, W. W. et al. Prevalence of acquired undescended testis in 6-year, 9-year and 13-year-old Dutch schoolboys. Arch. Dis. Child. 92, 17–20 (2007).
Wohlfahrt-Veje, C. et al. Acquired cryptorchidism is frequent in infancy and childhood. Int. J. Androl. 32, 423–428 (2009).
Ritzen, E. M. et al. Nordic consensus on treatment of undescended testes. Acta Pediatr. 96, 638–643 (2007).
Ashley, R. A., Barthold, J. S. & Kolon, T. F. Cryptorchidism: pathogenesis, diagnosis, treatment and prognosis. Urol. Clin. North Am. 37, 183–193 (2010).
Biggs, M. L., Baer, A. & Critchlow, C. W. Maternal, delivery, and perinatal characteristics associated with cryptorchidism: a population-based case-control study among births in Washington State. Epidemiology 13, 197–204 (2002).
Virtanen, H. E. et al. Mild gestational diabetes as a risk factor for congenital cryptorchidism. J. Clin. Endocrinol. Metab. 91, 4862–4865 (2006).
Weidner, I. S., Moller, H., Jensen, T. K. & Skakkebaek, N. E. Risk factors for cryptorchidism and hypospadias. J. Urol. 161, 1606–1609 (1999).
Schnack, T. H. et al. Familial aggregation of cryptorchidism—a nationwide cohort study. Am. J. Epidemiol. 167, 1453–1457 (2008).
Jensen, M. S. et al. Cryptorchidism concordance in monozygotic and dizygotic twin brothers, full brothers, and half-brothers. Fertil. Steril. 93, 124–129 (2010).
Sohval, A. R. Testicular dysgenesis as an etiologic factor in cryptorchidism. J. Urol. 72, 693–702 (1954).
Giwercman, A., Bruun, E., Frimodt-Moller, C. & Skakkebaek, N. E. Prevalence of carcinoma in situ and other histopathological abnormalities in testes of men with a history of cryptorchidism. J. Urol. 142, 998–1001 (1989).
Skakkebaek, N. E., Rajpert-De Meyts, E. & Main, K. M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 (2001).
Holm, M., Rajpert-De Meyts, E., Andersson, A. M. & Skakkebaek, N. E. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J. Pathol. 199, 378–386 (2003).
Nielsen, H., Nielsen, M. & Skakkebaek, N. E. The fine structure of possible carcinoma in situ in the seminiferous tubules in the testis of four infertile men. Acta Pathol. Microbiol. Scand. A. 82, 235–248 (1974).
Rajpert-De Meyts, E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum. Reprod. Update 12, 303–323 (2006).
Jorgensen, N., Meyts, E. R., Main, K. M. & Skakkebaek, N. E. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int. J. Androl. 33, 298–303 (2010).
Schnack, T. H., Poulsen, G., Myrup, C., Wohlfahrt, J. & Melbye, M. Familial coaggregation of cryptorchidism and hypospadias. Epidemiology 21, 109–113 (2010).
Clemmesen, J. A doubling of morbidity from testis carcinoma in Copenhagen, 1943–1962. Acta Pathol. Microbiol. Scand. 72, 348–349 (1968).
Richiardi, L. et al. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol. Biomarkers Prev. 13, 2157–2166 (2004).
Huyghe, E., Matsuda, T. & Thonneau, P. Increasing incidence of testicular cancer worldwide: a review. J. Urol. 170, 5–11 (2003).
Carlsen, E., Giwercman, A., Keiding, N. & Skakkebaek, N. E. Evidence for decreasing quality of semen during past 50 years. BMJ 305, 609–613 (1992).
Auger, J., Kunstmann, J. M., Czyglik, F. & Jouannet, P. Decline in semen quality among fertile men in Paris during the past 20 years. N. Engl. J. Med. 332, 281–285 (1995).
Swan, S. H., Elkin, E. P. & Fenster, L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 108, 961–966 (2000).
Lund, L. et al. Prevalence of hypospadias in Danish boys: a longitudinal study, 1977–2005. Eur. Urol. 55, 1022–1026 (2009).
Boisen, K. et al. Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at 3 months of age. J. Clin. Endocrinol. Metab. 90, 4041–4046 (2005).
Jorgensen, N. et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum. Reprod. 17, 2199–2208 (2002).
Hemminki, K. & Li, X. Cancer risks in Nordic immigrants and their offspring in Sweden. Eur. J. Cancer 38, 2428–2434 (2002).
Myrup, C. et al. Testicular cancer risk in first- and second-generation immigrants to Denmark. J. Natl. Cancer Inst. 100, 41–47 (2008).
Toppari, J. et al. Male reproductive health and environmental xenestrogens. Environ. Health Perspect. 104, 741–803 (1996).
Gray, L. E. et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update 7, 248–264 (2001).
Emmen, J. M. et al. Involvement of insulin-like factor 3 (INSL3) in diethylstilbestrol-induced cryptorchidism. Endocrinology 141, 846–849 (2000).
Nef, S., Shipman, T. & Parada, L. F. A molecular basis for estrogen-induced cryptorchidism. Dev. Biol. 224, 354–361 (2000).
Sharpe, R. M. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract. Res. Clin. Endocrinol. Metab. 20, 91–110 (2006).
Fisher, J. S., Macpherson, S., Marchetti, N. & Sharpe, R. M. Human 'testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 18, 1383–1394 (2003).
Kristensen, P., Irgens, L. M., Andersen, A., Bye, A. S. & Sundheim, L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology 8, 537–544 (1997).
Weidner, I. S., Moller, H., Jensen, T. K. & Skakkebaek, N. E. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ. Health Perspect. 106, 793–796 (1998).
Fernandez, M. F. et al. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: a nested case-control study. Environ. Health Perspect. 115 (Suppl. 1), 8–14 (2007).
Andersen, H. R. et al. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ. Health Perspect. 116, 566–572 (2008).
Damgaard, I. N. et al. Persistent pesticides in human breast milk and cryptorchidism. Environ. Health Perspect. 114, 1133–1138 (2006).
Pierik, F. H., Klebanoff, M. A., Brock, J. W. & Longnecker, M. P. Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and beta-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environ. Res. 105, 364–369 (2007).
Main, K. M. et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ. Health Perspect. 115, 1519–1526 (2007).
Small, C. M. et al. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ. Health Perspect. 117, 1175–1179 (2009).
McGlynn, K. A. et al. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ. Health Perspect. 117, 1472–1476 (2009).
Jensen, M. S. et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology 21, 779–785 (2010).
Kristensen, D. M. et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum. Reprod. 26, 235–244 (2011).
Akre, O., Lipworth, L., Cnattingius, S., Sparen, P. & Ekbom, A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology 10, 364–369 (1999).
Jensen, M. S., Toft, G., Thulstrup, A. M., Bonde, J. P. & Olsen, J. Cryptorchidism according to maternal gestational smoking. Epidemiology 18, 220–225 (2007).
Thorup, J., Cortes, D. & Petersen, B. L. The incidence of bilateral cryptorchidism is increased and the fertility potential is reduced in sons born to mothers who have smoked during pregnancy. J. Urol. 176, 734–737 (2006).
Damgaard, I. N. et al. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLoS One 3, e3051 (2008).
Damgaard, I. N. et al. Cryptorchidism and maternal alcohol consumption during pregnancy. Environ. Health Perspect. 115, 272–277 (2007).
Jensen, M. S., Bonde, J. P. & Olsen, J. Prenatal alcohol exposure and cryptorchidism. Acta Pediatr. 96, 1681–1685 (2007).
Strandberg-Larsen, K., Jensen, M. S., Ramlau-Hansen, C. H., Gronbaek, M. & Olsen, J. Alcohol binge drinking during pregnancy and cryptorchidism. Hum. Reprod. 24, 3211–3219 (2009).
Savion, M., Nissenkorn, I., Servadio, C. & Dickerman, Z. Familial occurrence of undescended testes. Urology 23, 355–358 (1984).
La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E. & Fischbeck, K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Aschim, E. L. et al. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. J. Clin. Endocrinol. Metab. 89, 5105–5109 (2004).
Castro-Nallar, E. et al. Androgen receptor gene cag and ggn repeat polymorphisms in Chilean men with primary severe spermatogenic failure. J. Androl. 31, 552–559 (2010).
Ferlin, A. et al. Androgen receptor gene CAG and GGC repeat lengths in cryptorchidism. Eur. J. Endocrinol. 152, 419–425 (2005).
Lim, H. N., Nixon, R. M., Chen, H., Hughes, I. A. & Hawkins, J. R. Evidence that longer androgen receptor polyglutamine repeats are a causal factor for genital abnormalities. J. Clin. Endocrinol. Metab. 86, 3207–3210 (2001).
Sasagawa, I. et al. CAG repeat length of the androgen receptor gene in Japanese males with cryptorchidism. Mol. Hum. Reprod. 6, 973–975 (2000).
Silva-Ramos, M. et al. The CAG repeat within the androgen receptor gene and its relationship to cryptorchidism. Int. Braz. J. Urol. 32, 330–334 (2006).
Koskimies, P. et al. A common polymorphism in the human relaxin-like factor (RLF) gene: no relationship with cryptorchidism. Pediatr. Res. 47, 538–541 (2000).
Lim, H. N., Rajpert-De Meyts, E., Skakkebæk, N. E., Hawkins, J. R. & Hughes, I. A. Genetic analysis of the INSL3 gene in patients with maldescent of the testis. Eur. J. Endocrinol. 144, 129–137 (2001).
Roh, J. et al. Lack of LGR8 gene mutation in Finnish patients with a family history of cryptorchidism. Reprod. Biomed. Online 7, 400–406 (2003).
Feng, S. et al. Mutation analysis of INSL3 and GREAT/LGR8 genes in familial cryptorchidism. Urology 64, 1032–1036 (2004).
Bogatcheva, N. V. et al. T222P mutation of the insulin-like 3 hormone receptor LGR8 is associated with testicular maldescent and hinders receptor expression on the cell surface membrane. Am. J. Physiol. Endocrinol. Metab. 292, E138–E144 (2007).
Foresta, C., Zuccarello, D., Garolla, A. & Ferlin, A. Role of hormones, genes, and environment in human cryptorchidism. Endocr. Rev. 29, 560–580 (2008).
Ferlin, A. et al. Genetic alterations associated with cryptorchidism. JAMA 300, 2271–2276 (2008).
El Houate, B. et al. No association between T222P/LGR8 mutation and cryptorchidism in the Moroccan population. Horm. Res. 70, 236–239 (2008).
Nuti, F. et al. The leucine-rich repeat-containing G protein-coupled receptor 8 gene T222P mutation does not cause cryptorchidism. J. Clin. Endocrinol. Metab. 93, 1072–1076 (2008).
Ars, E. et al. Further insights into the role of T222P variant of RXFP2 in non-syndromic cryptorchidism in two Mediterranean populations. Int. J. Androl. doi:10.1111/j.1365–26052010.01088.x.
Kolon, T. F. et al. Analysis of homeobox gene HOXA10 mutations in cryptorchidism. J. Urol. 161, 275–280 (1999).
Bertini, V. et al. Homeobox HOXA10 gene analysis in cryptorchidism. J. Pediatr. Endocrinol. Metab. 17, 41–45 (2004).
Gottlieb, B., Beitel, L. K. & Trifiro, M. A. Androgen insensitivity syndrome. In Gene Reviews [online] (eds Pagon, R. A., Bird, T. C., Dolan, C. R. & Stephens, K.) (University of Washington, Seattle, 1999).
Lanfranco, F., Kamischke, A., Zitzmann, M. & Nieschlag, E. Klinefelter's syndrome. Lancet 364, 273–283 (2004).
Quinton, R. et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin. Endocrinol. (Oxf.) 55, 163–174 (2001).
Wu, S. M. & Chan, W. Y. Male pseudohermaphroditism due to inactivating luteinizing hormone receptor mutations. Arch. Med. Res. 30, 495–500 (1999).
Drake, A. J. et al. Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology 150, 5055–5064 (2009).
Krysiak-Baltyn, K. et al. Country-specific chemical signatures of persistent environmental compounds in breast milk. Int. J. Androl. 33, 270–278 (2010).
Watanabe, M. et al. Haplotype analysis of the estrogen receptor 1 gene in male genital and reproductive abnormalities. Hum. Reprod. 22, 1279–1284 (2007).
Galan, J. J. et al. Molecular analysis of estrogen receptor alpha gene AGATA haplotype and SNP12 in European populations: potential protective effect for cryptorchidism and lack of association with male infertility. Hum. Reprod. 22, 444–449 (2007).
Audouze, K. et al. Deciphering diseases and biological targets for environmental chemicals using toxicogenomics networks. PLoS. Comput. Biol. 20, e1000788 (2010).
Acknowledgements
C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of content and reviewed and edited the article before submission. K. Bay and J. Toppari wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bay, K., Main, K., Toppari, J. et al. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nat Rev Urol 8, 187–196 (2011). https://doi.org/10.1038/nrurol.2011.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.23
This article is cited by
-
Genetic control of typical and atypical sex development
Nature Reviews Urology (2023)
-
Hormone measurements and histomorphological observations in male Bactrian camels
Tropical Animal Health and Production (2023)
-
Association of PFKM gene polymorphisms and susceptibility to cryptorchidism in a Chinese Han population
Pediatric Surgery International (2022)
-
Insulin-like 3 affects zebrafish spermatogenic cells directly and via Sertoli cells
Communications Biology (2021)
-
An approach to classifying occupational exposures to endocrine disrupting chemicals by sex hormone function using an expert judgment process
Journal of Exposure Science & Environmental Epidemiology (2021)