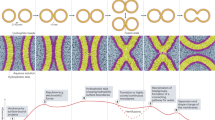
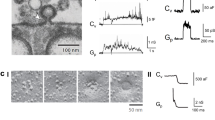
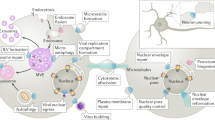
Abstract
Subcellular compartmentalization, cell growth, hormone secretion and neurotransmission require rapid, targeted, and regulated membrane fusion. Fusion entails extensive lipid rearrangements by two apposed (docked) membrane vesicles, joining their membrane proteins and lipids and mixing their luminal contents without lysis. Fusion of membranes in the secretory pathway involves Rab GTPases; their bound 'effector' proteins, which mediate downstream steps; SNARE proteins, which can 'snare' each other, in cis (bound to one membrane) or in trans (anchored to apposed membranes); and SNARE-associated proteins (SM proteins; NSF or Sec18p; SNAP or Sec17p; and others) cooperating with specific lipids to catalyze fusion. In contrast, mitochondrial and cell-cell fusion events are regulated by and use distinct catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chernomordik, L.V. & Kozlov, M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 15, 675–683 (2008).
Malinin, V.S., Frederik, P. & Lentz, B.R. Osmotic and curvature stress affect PEG-induced fusion of lipid vesicles but not mixing of their lipids. Biophys. J. 82, 2090–2100 (2002).
Siegel, D.P. et al. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry 38, 3703–3709 (1989).
Burgess, S.W., McIntosh, T.J. & Lentz, B.R. Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry 31, 2653–2661 (1992).
Lee, J. & Lentz, B.R. Evolution of lipidic structures during model membrane fusion and the relation of this process to cell membrane fusion. Biochemistry 36, 6251–6259 (1997).
Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 15, 690–698 (2008).
Chernomordik, L.V., Frolov, V.A., Leikina, E., Bronk, P. & Zimmerberg, J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140, 1369–1382 (1998).
Melikyan, G.B., Brener, S.A., Cok, D. & Cohen, F.S. Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion. J. Cell Biol. 136, 995–1005 (1997).
Wharton, S.A., Martin, S.R., Ruigrok, R.W.H., Skehel, J.J. & Wiley, D.C. Membrane fusion by peptide analogues of influenza haemagglutinin. J. Gen. Virol. 69, 1847–1857 (1988).
Frolov, V.A., Dunina-Barkovskaya, A.Y., Samsonov, A.V. & Zimmerberg, J. Membrane permeability changes at early stages of Influenza hemagglutinin-mediated fusion. Biophys. J. 85, 1725–1733 (2003).
Skehel, J.J. & Wiley, D.C. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell 95, 871–874 (1998).
Beisson, J., Lefort-Tran, M., Pouphile, M., Rossignol, M. & Satir, B. Genetic analysis of membrane differentiation in Paramecium. Freeze-fracture study of the trichocyst cycle in wild-type and mutant strains. J. Cell Biol. 69, 126–143 (1976).
Novick, P. & Schekman, R. Secretion and cell-surface growth are blocked in a temperature sensitive mutant of Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA 76, 1858–1862 (1979).
Novick, P., Field, C. & Schekman, R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 (1980).
Kaiser, C.A. & Schekman, R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61, 723–733 (1990).
Fries, E. & Rothman, J.E. Transport of vesicular stomatitis virus glycoprotein in a cell-free extract. Proc. Natl. Acad. Sci. USA 77, 3870–3874 (1980).
Baker, D., Hicke, L., Rexach, M., Schleyer, M. & Schekman, R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54, 335–344 (1988).
Rothman, J.H. & Stevens, T.H. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell 47, 1041–1051 (1986).
Banta, L.M., Robinson, J.S., Klionsky, D.J. & Emr, S.D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107, 1369–1383 (1988).
Wada, Y., Ohsumi, Y. & Anraku, Y. Genes for directing vacuole morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem. 267, 18665–18670 (1992).
Wilson, D.W. et al. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature 339, 355–359 (1989).
Südhof, T. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Trimble, W.S., Cowan, D.M. & Scheller, R.H. VAMP-1: A synaptic vesicle-associated integral membrane protein. Proc. Natl. Acad. Sci. USA 85, 4538–4542 (1988).
Baumert, M., Maycox, P.R., Navone, F., De Camilli, P. & Jahn, R. Synaptobrevin: an integral membrane protein of 18 000 daltons present in small synaptic vesicles of rat brain. EMBO J. 8, 379–384 (1989).
Bennett, M.K., Calakos, N. & Scheller, R.H. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257, 255–259 (1992).
Schiavo, G. et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359, 832–835 (1992).
Rizo, J., Chen, X. & Arac, D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 16, 339–350 (2006).
Tokumaru, H. et al. SNARE complex oligomerization by Spaphin/Complexin is essential for synaptic vesicle exocytosis. Cell 104, 421–432 (2001).
Roggero, C.M. et al. Complexin/synaptotagmin interplay controls acrosomal exocytosis. J. Biol. Chem. 282, 26335–26343 (2007).
Söllner, T., Bennett, M.K., Whiteheart, S.W., Scheller, R.H. & Rothman, J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 (1993).
Söllner, T. et al. SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 (1993).
Fasshauer, D., Sutton, R.B., Brunger, A.T. & Jahn, R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 95, 15781–15786 (1998).
Rickman, C. & Davletov, B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J. Biol. Chem. 278, 5501–5504 (2003).
Tang, J. et al. A complexin/synaptotagmin1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 (2006).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Bhalla, A., Chicka, M.C., Tucker, W.C. & Chapman, E.R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006).
Shen, J., Tareste, D.C., Paumet, F., Rothman, J.E. & Mella, T.J. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 (2007).
Block, M.R., Glick, B.S., Wilcox, C.A., Wieland, F.T. & Rothman, J.E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl. Acad. Sci. USA 85, 7852–7856 (1988).
Clary, D.O. & Rothman, J.E. Purification of three related peripheral membrane proteins needed for vesicular transport. J. Biol. Chem. 265, 10109–10117 (1990).
Rice, L.M. & Brunger, A.T. Crystal structure of the vesicular transport protein Sec17: Implications for SNAP function in SNARE complex disassembly. Mol. Cell 4, 85–95 (1999).
Cheever, M.L. et al. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 3, 613–618 (2001).
Lang, T. et al. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 20, 2202–2213 (2001).
Wang, L., Seeley, S., Wickner, W. & Merz, A. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell 108, 357–369 (2002).
Collins, K.M. & Wickner, W.T. trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc. Natl. Acad. Sci. USA 104, 8755–8760 (2007).
Nichols, B.J., Ungermann, C., Pelham, H.R.B., Wickner, W.T. & Haas, A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387, 199–202 (1997).
Cho, S.-J. et al. SNAREs in opposing bilayers interact in a circular array to form conducting pores. Biophys. J. 83, 2522–2527 (2002).
Rizo, J. & Südhof, T.C. SNAREs and Munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 3, 641–653 (2002).
Carr, C.M., Grote, E., Munson, M., Hughson, F.M. & Novick, P.J. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146, 333–344 (1999).
Scott, B.L. et al. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro . J. Cell Biol. 167, 75–85 (2004).
Dulubova, I. et al. How Tlg2p/syntaxin 16 'snares' Vps45. EMBO J. 21, 3620–3631 (2002).
Yamaguchi, T. et al. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev. Cell 2, 295–305 (2002).
Dulubova, I., Sugita, S., Hill, S., Hosaka, M. & Fernandez, I. Südhof, T.C., & Rizo, J. A conformational switch in syntaxin during exocytosis: Role of Munc18. EMBO J. 18, 4372–4382 (1999).
Misura, K.M., Scheller, R.H. & Weis, W.I. Three-dimensional structure of the neuronal Sec1-syntaxin 1a complex. Nature 404, 355–362 (2000).
Peng, R. & Gallwitz, D. Sly1 protein bound to Golgi syntaxin Sec5 allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol. 157, 645–655 (2002).
Goud, B., Salminen, A., Walworth, N.C. & Novick, P.J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53, 753–768 (1988).
Walworth, N.C., Goud, B., Kabcenell, A.K. & Novick, P.J. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 8, 1685–1693 (1989).
Collins, R.N., Brennwald, P., Garrett, M., Lauring, A. & Novick, P. Interactions of nucleotide release factor Dss4p with Sec4p in the post-Golgi secretory pathway of yeast. J. Biol. Chem. 272, 18281–18289 (1997).
TerBush, D.R., Maurice, T., Roth, D. & Novick, P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae . EMBO J. 15, 6483–6494 (1996).
Finger, F.P. & Novick, P. Spatial regulation of exocytosis; lessons from yeast. J. Cell Biol. 142, 609–612 (1998).
Guo, W., Roth, D., Walch-Solumena, C. & Novick, P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071–1080 (1999).
Sacher, M. et al. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 17, 2494–2503 (1998).
Stroupe, C., Collins, K.M., Fratti, R.A. & Wickner, W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 25, 1579–1589 (2006).
Wurmser, A.E., Sato, T.K. & Emr, S.D. New component of the vacuolar class C–Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J. Cell Biol. 151, 551–562 (2000).
Wang, W., Sacher, M. & Ferro-Novick, S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J. Cell Biol. 151, 289–295 (2000).
Medkova, M., France, Y.E., Coleman, J. & Novick, P. The rab exchange factor Sec1p reversibly associates with the exocyst. Mol. Biol. Cell 17, 2757–2769 (2006).
Stenmark, H. et al. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287–1296 (1994).
Stenmark, H., Vitale, G., Ullrich, O. & Zerial, M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 83, 423–432 (1995).
Horiuchi, H. et al. A novel Rab5 GDP/GTP exchange factor complexed to rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159 (1997).
Christoforidis, S., McBride, H.M., Burgoyne, R.D. & Zerial, M. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621–625 (1999a).
Christoforidis, S. et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1, 249–252 (1999).
De Renzis, S., Sönnichsen, B. & Zerial, M. Divalent Rab effectors regulate the subcompartmental organization and sorting of early endosomes. Nat. Cell Biol. 4, 124–133 (2002).
Simonsen, A. et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494–498 (1998).
Miaczynska, M. & Zerial, M. Mosaic organization of the endocytic pathway. Exp. Cell Res. 272, 8–14 (2002).
Lippe, R., Miaczynska, M., Rybin, V., Runge, A. & Zerial, M. Functional synergy between Rab5 effector rabaptin-5 and exchange factor rabex-5 when physically associated in a complex. Mol. Biol. Cell 12, 2219–2228 (2001).
Shin, H.-W. et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 170, 607–618 (2005).
McBride, H.M. et al. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98, 377–386 (1999).
Wang, L., Merz, A., Collins, K. & Wickner, W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J. Cell Biol. 160, 365–374 (2003).
Fratti, R., Jun, Y., Merz, A.J., Margolis, N. & Wickner, W. Interdependent assembly of specific “regulatory” lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J. Cell Biol. 167, 1087–1098 (2004).
Jun, Y. et al. Reversible, cooperative reactions of yeast vacuole docking. EMBO J. 25, 5260–5269 (2006).
Struck, D.K., Hoekstra, D. & Pagano, R.E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20, 4093–4099 (1981).
Düzgünes, N., Allen, T.M., Fedor, J. & Papahadjopoulos, D. Lipid mixing during membrane aggregation and fusion: Why fusion assays disagree. Biochemistry 26, 8435–8442 (1987).
Meers, P., Ali, S., Erukulla, R. & Janoff, A.S. Novel inner monolayer fusion assays reveal differential monolayer mixing associated with cation-dependent membrane fusion. Biochim. Biophys. Acta 1467, 227–243 (2000).
McNew, J.A. et al. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159 (2000).
Zwilling, D. et al. Early endosomal SNAREs form a structurally conserved SNARE complex and fuse liposomes with multiple topologies. EMBO J. 26, 9–18 (2007).
Xu, Y., Zhang, F., Su, Z., McNew, J.A. & Shin, Y.-K. Hemifusion in SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 12, 417–422 (2005).
McNew, J.A., Weber, T., Engelman, D.M., Söllner, T.H. & Rothman, J.E. The length of the flexible SNAREpin juxtamembrane region is a critical determinant of SNARE-dependent fusion. Mol. Cell 4, 415–421 (1999).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Nickel, W. et al. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc. Natl. Acad. Sci. USA 96, 12571–12576 (1999).
Chen, X. et al. SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys. J. 90, 2062–2074 (2006).
Dennison, S.M., Bowen, M.E., Brunger, A.T. & Lentz, B.R. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys. J. 90, 1661–1675 (2006).
Starai, V., Jun, Y. & Wickner, W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc. Natl. Acad. Sci. USA 104, 13551–13558 (2007).
Jun, Y., Xu, H., Thorngren, N. & Wickner, W. Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion. EMBO J. 26, 4935–4945 (2007).
Melia, T.J., You, D., Tareste, D.C. & Rothman, J.E. Lipidic antagonists to SNARE-mediated fusion. J. Biol. Chem. 281, 29597–29605 (2006).
Hirling, H. & Scheller, R. Phosphorylation of synaptic vesicle proteins: Modulation of the aSNAP interaction with the core complex. Proc. Natl. Acad. Sci. USA 93, 11945–11949 (1996).
Fujita, Y. et al. Phosphorylation of Munc-18/nSec1/rbSec1 by protein kinase C. J. Biol. Chem. 271, 7265–7268 (1996).
LaGrassa, T.J. & Ungermann, C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J. Cell Biol. 168, 401–414 (2005).
Cao, X., Ballew, N. & Barlowe, C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17, 2156–2165 (1998).
Shorter, J., Beard, M.B., Seemann, J., Dirac-Svejstrup, A.B. & Warren, G. Sequential tethering of golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45–62 (2002).
Nakamura, N., Hirata, A., Ohsumi, Y. & Wada, Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membrane and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae . J. Biol. Chem. 272, 11344–11349 (1997).
Langosch, D. et al. Conformation of the synaptobrevin transmembrane domain. J. Mol. Biol. 311, 709–721 (2001).
Hofmann, M.W. et al. Self-interaction of a SNARE transmembrane domain promotes the hemifusion to fusion transition. J. Mol. Biol. 364, 1048–1060 (2006).
Siegel, D.P. et al. Transmembrane peptides stabilize inverted cubic phases in a biphasic length-dependent manner: implications for protein-induced membrane fusion. Biophys. J. 90, 200–211 (2006).
Bowen, M. & Brunger, A.T. Conformation of the synaptobrevin transmembrane domain. Proc. Natl. Acad. Sci. USA 103, 8378–8383 (2006).
Das, S. & Rand, R.P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem. Biophys. Res. Commun. 124, 491–496 (1984).
Hales, K.G. & Fuller, M.T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 90, 121–129 (1997).
Hermann, G.J., Thatcher, J.W. & Mills, J.P. Hales, K.G., Fuller, M.T., Nunnari, J., & Shaw, J.M. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 143, 359–373 (1998).
Rapaport, D., Brunner, M., Neupert, W. & Westermann, B. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae . J. Biol. Chem. 273, 20150–20155 (1998).
Koshiba, T. et al. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305, 858–862 (2004).
Meeusen, S., McCaffery, J.M. & Nunnari, J. Mitochondrial fusion intermediates revealed in vitro. Science 305, 1747–1752 (2004).
Sesaki, H. & Jensen, R.E. Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion. J. Biol. Chem. 279, 28298–28303 (2004).
Wong, E.D. et al. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 151, 341–352 (2000).
Meeusen, S. et al. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell 127, 383–395 (2006).
Mohler, W.A. et al. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev. Cell 2, 355–362 (2002).
Sapir, A. et al. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans . Dev. Cell 12, 683–698 (2007).
Podbilewicz, B. et al. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev. Cell 11, 471–481 (2006).
Shemer, G. et al. EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans . Curr. Biol. 14, 1587–1591 (2004).
del Campo, J.J. et al. Fusogenic activity of EFF-1 is regulated via dynamic localization in fusing somatic cells of C. elegans . Curr. Biol. 15, 413–423 (2005).
McCaffrey, G., Clay, F.J., Kelsay, K. & Sprague, G.F. Jr. Identification and regulation of a gene required for cell fusion during mating of the yeast Saccharomyces cerevisiae . Mol. Cell. Biol. 7, 2680–2690 (1987).
Elion, E.A., Trueheart, J. & Fink, G.R. Fus2 localizes near the site of cell fusion and is required for both cell fusion and nuclear alignment during zygote formation. J. Cell Biol. 130, 1283–1296 (1995).
Gammie, A.E., Brizzio, V. & Rose, M.D. Distinct morphological phenotypes of cell fusion mutants. Mol. Biol. Cell 9, 1395–1410 (1998).
Heiman, M.G. & Walter, P. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 151, 719–730 (2000).
Jin, H., Carlile, C., Nolan, S. & Grote, E. Prm1 prevents contact-dependent lysis of yeast mating pairs. Eukaryot. Cell 3, 1664–1673 (2004).
Grosshans, B.L., Ortiz, D. & Novick, P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 103, 11821–11827 (2006).
Sönnichsen, B. et al. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140, 1013–1021 (1998).
Barr, F.A., Nakamura, N. & Warren, G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 17, 3258–3268 (1998).
Jahn, R. & Scheller, R. SNAREs — engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–646 (2006).
Acknowledgements
We thank J. Shaw, A. Engel and P. Walter for discussions. Work in the authors' laboratories was supported by grants from the US National Institutes of Health and funds from the Howard Hughes Medical Institute (R.S.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wickner, W., Schekman, R. Membrane fusion. Nat Struct Mol Biol 15, 658–664 (2008). https://doi.org/10.1038/nsmb.1451
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1451
This article is cited by
-
Mechanisms of SNARE proteins in membrane fusion
Nature Reviews Molecular Cell Biology (2024)
-
Extreme parsimony in ATP consumption by 20S complexes in the global disassembly of single SNARE complexes
Nature Communications (2021)
-
Free energies of membrane stalk formation from a lipidomics perspective
Nature Communications (2021)
-
Developing Nanodisc-ID for label-free characterizations of membrane proteins
Communications Biology (2021)
-
The Habc domain of syntaxin 3 is a ubiquitin binding domain
Scientific Reports (2020)