Abstract
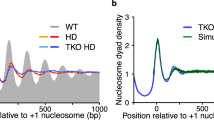
We assess the role of intrinsic histone-DNA interactions by mapping nucleosomes assembled in vitro on genomic DNA. Nucleosomes strongly prefer yeast DNA over Escherichia coli DNA, indicating that the yeast genome evolved to favor nucleosome formation. Many yeast promoter and terminator regions intrinsically disfavor nucleosome formation, and nucleosomes assembled in vitro show strong rotational positioning. Nucleosome arrays generated by the ACF assembly factor have fewer nucleosome-free regions, reduced rotational positioning and less translational positioning than obtained by intrinsic histone-DNA interactions. Notably, nucleosomes assembled in vitro have only a limited preference for specific translational positions and do not show the pattern observed in vivo. Our results argue against a genomic code for nucleosome positioning, and they suggest that the nucleosomal pattern in coding regions arises primarily from statistical positioning from a barrier near the promoter that involves some aspect of transcriptional initiation by RNA polymerase II.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Kornberg, R.D. Structure of chromatin. Annu. Rev. Biochem. 46, 931–954 (1977).
Yuan, G.-C. et al. Genome-scale identification of nucleosome positions in S. cerevisiae. Science 309, 626–630 (2005).
Lee, W. et al. A high-resolution atlas of nucleosome occupancy in yeast. Nat. Genet. 39, 1235–1244 (2007).
Mavrich, T.N. et al. Nucleosome organization in the Drosophila genome. Nature 453, 358–362 (2008).
Schones, D.E. et al. Dynamic regulation of nucleosome positioning in the human genome. Cell 132, 887–898 (2008).
Shivaswamy, S. et al. Dynamic remodeling of individual nucleosomes across a eukaryotic genome in response to transcriptional perturbation. PLoS Biol. 6, e65 (2008).
Valouev, A. et al. A high-resolution, nucleosome position map of C. elegans reveals a lack of universal sequence-dictated positioning. Genome Res. 18, 1051–1063 (2008).
Mavrich, T.N. et al. A barrier nucleosome model for statistical positioning of nucleosome throughout the yeast genome. Genome Res. 18, 1073–1083 (2008).
Deckert, J. & Struhl, K. Histone acetylation at promoters is differentially affected by activators and repressors. Mol. Cell. Biol. 21, 2726–2735 (2001).
Boeger, H. et al. Nucleosomes unfold completely at a transcriptionally active promoter. Mol. Cell 11, 1587–1598 (2003).
Reinke, H. & Horz, W. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell 11, 1599–1607 (2003).
Schwabish, M.A. & Struhl, K. The Swi/Snf complex is important for histone eviction during transcriptional activation and RNA polymerase II elongation in vivo. Mol. Cell. Biol. 27, 6987–6995 (2007).
Kristjuhan, A. & Svejstrup, J.Q. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J. 23, 4243–4252 (2004).
Lee, C.K. et al. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 36, 900–905 (2004).
Schwabish, M.A. & Struhl, K. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 24, 10111–10117 (2004).
Kaplan, N. et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 458, 362–366 (2009).
Sekinger, E.A., Moqtaderi, Z. & Struhl, K. Intrinsic histone-DNA interactions and low nucleosome density are important for preferential accessibility of promoter regions in yeast. Mol. Cell 18, 735–748 (2005).
Iyer, V. & Struhl, K. Poly(dA:dT), a ubiquitous promoter element that stimulates transcription via its intrinsic structure. EMBO J. 14, 2570–2579 (1995).
Drew, H.R. & Travers, A.A. DNA bending and its relation to nucleosome positioning. J. Mol. Biol. 186, 773–790 (1985).
Satchwell, S.C., Drew, H.R. & Travers, A.A. Sequence periodicities in chicken nucleosome core DNA. J. Mol. Biol. 191, 659–675 (1986).
Widom, J. Role of DNA sequence in nucleosome stability and dynamics. Q. Rev. Biophys. 34, 269–324 (2001).
Fedor, M.J., Lue, N.F. & Kornberg, R.D. Statistical positioning of nucleosomes by specific protein binding to an upstream activating sequence in yeast. J. Mol. Biol. 204, 109–127 (1988).
Lowary, P.T. & Widom, J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276, 19–42 (1998).
Segal, E. et al. A genomic code for nucleosome positioning. Nature 442, 772–778 (2006).
Whitehouse, I. & Tsukiyama, T. Antagonistic forces that position nucleosomes in vivo. Nat. Struct. Mol. Biol. 13, 633–640 (2006).
Yang, A. et al. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol. Cell 24, 593–602 (2006).
Liu, X. et al. Whole-genome comparison of Leu3 binding in vitro and in vivo reveals the importance of nucleosome occupancy in target site selection. Genome Res. 16, 1517–1528 (2006).
Hörz, W. & Altenburger, W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 9, 2643–2658 (1981).
Struhl, K. Yeast transcriptional regulatory mechanisms. Annu. Rev. Genet. 29, 651–674 (1995).
Muse, G.W. et al. RNA polymerase is poised for activation across the genome. Nat. Genet. 39, 1507–1511 (2007).
Steinmetz, E.J. et al. Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol. Cell 24, 735–746 (2006).
Struhl, K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat. Struct. Mol. Biol. 14, 103–105 (2007).
Dion, M.F. et al. Dynamics of replication-independent histone turnover in budding yeast. Science 315, 1405–1408 (2007).
Fyodorov, D.V. & Kadonaga, J.T. Chromatin assembly in vitro with purified recombinant ACF and NAP-1. Methods Enzymol. 371, 499–515 (2003).
Acknowledgements
We thank I. Albert, Z. Zhang and F. Pugh for analyses and commentary during the early stages of this work, and Y. Lei and H. Shin for help with the heat maps and power spectrum analysis, respectively. This work was supported by grants to J.T.K. (GM 58272), X.S.L. (HG 4069) and K.S. (GM 30186) from the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Y.Z. performed all the bioinformatic analyses and contributed to writing of the manuscript; Z.M. prepared the genomic DNAs and the sonicated DNA sample, prepared the micrococcal nuclease–treated samples for DNA sequencing and contributed to the experimental design, data analysis and writing of the manuscript; B.P.R. assembled nucleosomes on genomic DNAs; J.T.K. contributed to experimental design and analysis of the nucleosome samples; G.E. sequenced the DNA samples with contributions from M.S.; X.S.L. contributed to the design and interpretation of the bioinformatic analyses and writing the manuscript; K.S. conceived of the project, contributed to the data analysis and wrote most of the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Methods (PDF 2627 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Moqtaderi, Z., Rattner, B. et al. Intrinsic histone-DNA interactions are not the major determinant of nucleosome positions in vivo. Nat Struct Mol Biol 16, 847–852 (2009). https://doi.org/10.1038/nsmb.1636
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1636
This article is cited by
-
Energy-driven genome regulation by ATP-dependent chromatin remodellers
Nature Reviews Molecular Cell Biology (2024)
-
Dynamic nucleosome organization after fertilization reveals regulatory factors for mouse zygotic genome activation
Cell Research (2022)
-
Ruler elements in chromatin remodelers set nucleosome array spacing and phasing
Nature Communications (2021)
-
Genome information processing by the INO80 chromatin remodeler positions nucleosomes
Nature Communications (2021)
-
DNA origami-based single-molecule force spectroscopy elucidates RNA Polymerase III pre-initiation complex stability
Nature Communications (2020)