Abstract
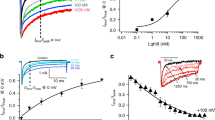
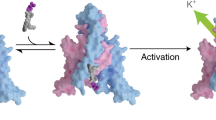
Voltage-activated ion channels open and close in response to changes in voltage, a property that is essential for generating nerve impulses. Studies on voltage-activated potassium (Kv) channels show that voltage-sensor activation is sensitive to the composition of lipids in the surrounding membrane. Here we explore the interaction of lipids with S1–S4 voltage-sensing domains and find that the conversion of the membrane lipid sphingomyelin to ceramide-1-phosphate alters voltage-sensor activation in an S1–S4 voltage-sensing protein lacking an associated pore domain, and that the S3b–S4 paddle motif determines the effects of lipid modification on Kv channels. Using tarantula toxins that bind to paddle motifs within the membrane, we identify mutations in the paddle motif that weaken toxin binding by disrupting lipid-paddle interactions. Our results suggest that lipids bind to voltage-sensing domains and demonstrate that the pharmacological sensitivities of voltage-activated ion channels are influenced by the surrounding lipid membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Long, S.B., Tao, X., Campbell, E.B. & MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007).
Doyle, D.A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Swartz, K.J. Sensing voltage across lipid membranes. Nature 456, 891–897 (2008).
Jiang, Y. et al. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41 (2003).
Schmidt, D., Jiang, Q.X. & MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 444, 775–779 (2006).
Ramu, Y., Xu, Y. & Lu, Z. Enzymatic activation of voltage-gated potassium channels. Nature 442, 696–699 (2006).
Xu, Y., Ramu, Y. & Lu, Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature 451, 826–829 (2008).
Alabi, A.A., Bahamonde, M.I., Jung, H.J., Kim, J.I. & Swartz, K.J. Portability of paddle motif function and pharmacology in voltage sensors. Nature 450, 370–375 (2007).
Milescu, M. et al. Tarantula toxins interact with voltage sensors within lipid membranes. J. Gen. Physiol. 130, 497–511 (2007).
Schmidt, D. & Mackinnon, R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc. Natl. Acad. Sci. USA 105, 19276–19281 (2008).
Kamb, A., Tseng-Crank, J. & Tanouye, M.A. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron 1, 421–430 (1988).
Frech, G.C., VanDongen, A.M., Schuster, G., Brown, A.M. & Joho, R.H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature 340, 642–645 (1989).
Palsdottir, H. & Hunte, C. Lipids in membrane protein structures. Biochim. Biophys. Acta 1666, 2–18 (2004).
Lee, A.G. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612, 1–40 (2003).
Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K. & Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243 (2005).
Villalba-Galea, C.A., Sandtner, W., Starace, D.M. & Bezanilla, F. S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. USA 105, 17600–17607 (2008).
Villalba-Galea, C.A., Miceli, F., Taglialatela, M. & Bezanilla, F. Coupling between the voltage-sensing and phosphatase domains of Ci-VSP. J. Gen. Physiol. 134, 5–14 (2009).
Cuello, L.G., Cortes, D.M. & Perozo, E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 306, 491–495 (2004).
Ruta, V., Chen, J. & MacKinnon, R. Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell 123, 463–475 (2005).
Banerjee, A. & MacKinnon, R. Inferred motions of the S3a helix during voltage-dependent K+ channel gating. J. Mol. Biol. 381, 569–580 (2008).
Bosmans, F., Martin-Eauclaire, M.F. & Swartz, K.J. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature 456, 202–208 (2008).
Chakrapani, S., Cuello, L.G., Cortes, D.M. & Perozo, E. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure 16, 398–409 (2008).
Trimmer, J.S. et al. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron 3, 33–49 (1989).
Lee, S.Y. & MacKinnon, R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430, 232–235 (2004).
Jung, H.J. et al. Solution structure and lipid membrane partitioning of VSTx1, an inhibitor of the KvAP potassium channel. Biochemistry 44, 6015–6023 (2005).
Phillips, L.R. et al. Voltage-sensor activation with a tarantula toxin as cargo. Nature 436, 857–860 (2005).
Swartz, K.J. & MacKinnon, R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18, 675–682 (1997b).
Li-Smerin, Y., Hackos, D.H. & Swartz, K.J. α-Helical structural elements within the voltage-sensing domains of a K+ channel. J. Gen. Physiol. 115, 33–50 (2000a).
Li-Smerin, Y. & Swartz, K.J. Helical structure of the COOH terminus of S3 and its contribution to the gating modifier toxin receptor in voltage-gated ion channels. J. Gen. Physiol. 117, 205–218 (2001).
Herrington, J. et al. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 55, 1034–1042 (2006).
Ruta, V., Jiang, Y., Lee, A., Chen, J. & MacKinnon, R. Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature 422, 180–185 (2003).
Middleton, R.E. et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry 41, 14734–14747 (2002).
Li-Smerin, Y. & Swartz, K.J. Localization and molecular determinants of the hanatoxin receptors on the voltage-sensing domain of a K+ channel. J. Gen. Physiol. 115, 673–684 (2000).
Hidalgo, P. & MacKinnon, R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science 268, 307–310 (1995).
Schreiber, G. & Fersht, A.R. Energetics of protein-protein interactions: analysis of the barnase-barstar interface by single mutations and double mutant cycles. J. Mol. Biol. 248, 478–486 (1995).
Martens, J.R. et al. Differential targeting of Shaker-like potassium channels to lipid rafts. J. Biol. Chem. 275, 7443–7446 (2000).
Nguyen, T.P. & Horn, R. Movement and crevices around a sodium channel S3 segment. J. Gen. Physiol. 120, 419–436 (2002).
Gandhi, C.S., Clark, E., Loots, E., Pralle, A. & Isacoff, E.Y. The orientation and molecular movement of a K+ channel voltage-sensing domain. Neuron 40, 515–525 (2003).
Darman, R.B., Ivy, A.A., Ketty, V. & Blaustein, R.O. Constraints on voltage sensor movement in the Shaker K+ channel. J. Gen. Physiol. 128, 687–699 (2006).
Soler-Llavina, G.J., Chang, T.H. & Swartz, K.J. Functional interactions at the interface between voltage-sensing and pore domains in the Shaker Kv channel. Neuron 52, 623–634 (2006).
Long, S.B., Campbell, E.B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Mahfoud, R. et al. Identification of a common sphingolipid-binding domain in Alzheimer, prion, and HIV-1 proteins. J. Biol. Chem. 277, 11292–11296 (2002).
Snook, C.F., Jones, J.A. & Hannun, Y.A. Sphingolipid-binding proteins. Biochim. Biophys. Acta 1761, 927–946 (2006).
Swartz, K.J. & MacKinnon, R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron 18, 665–673 (1997a).
Lee, H.C., Wang, J.M. & Swartz, K.J. Interaction between extracellular Hanatoxin and the resting conformation of the voltage-sensor paddle in Kv channels. Neuron 40, 527–536 (2003).
Acknowledgements
We thank M. Holmgren, J. Kumar, M. Mayer, J. Mindell, S. Silberberg and members of the Swartz laboratory for helpful discussions and the US National Institute of Neurological Disorders and Stroke (NINDS) DNA sequencing facility for DNA sequencing. We thank Y. Okamura (Okazaki Center for Integrative Biosciences) for providing Ci-VSP complementary DNA, Y. Xu and Z. Lu (University of Pennsylvania) for supplying recombinant SMaseD and W. Schmalhofer and M. Garcia (Merck Research Labs) for supplying 125I-GxTx-1E. This work was supported by the Intramural Research Program of the NINDS, National Insitutes of Health (NIH) (to K.J.S.) and by an NIH-FWO postdoctoral fellowship (to F.B.).
Author information
Authors and Affiliations
Contributions
M.M., F.B. and A.A.A. performed molecular biology and electrophysiology experiments, and S.L. synthesized GxTx-1E. All authors contributed to the study design and to writing the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–3 (PDF 5807 kb)
Rights and permissions
About this article
Cite this article
Milescu, M., Bosmans, F., Lee, S. et al. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat Struct Mol Biol 16, 1080–1085 (2009). https://doi.org/10.1038/nsmb.1679
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1679
This article is cited by
-
TRP Channels, Conformational Flexibility, and the Lipid Membrane
The Journal of Membrane Biology (2020)
-
Molecular Interactions between Tarantula Toxins and Low-Voltage-Activated Calcium Channels
Scientific Reports (2016)
-
TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action
Nature (2016)
-
Structural basis for the inhibition of voltage-dependent K+ channel by gating modifier toxin
Scientific Reports (2015)
-
Conformational Dynamics of Shaker-Type Kv1.1 Ion Channel in Open, Closed, and Two Mutated States
The Journal of Membrane Biology (2015)