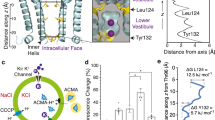
Abstract
Strong voltage sensitivity of inward-rectifier K+ (Kir) channels has been hypothesized to arise primarily from an intracellular blocker displacing up to five K+ ions from the wide, intracellular part of the ion conduction pore outwardly across the narrow ion-selectivity filter. The validity of this hypothesis depends on two assumptions: (i) that five ion sites are located intracellular to the filter and (ii) that the blocker can force essentially unidirectional K+ movement in a pore region generally wider than the combined dimensions of the blocker plus a K+ ion. Here we present a crystal structure of the cytoplasmic portion of a Kir channel with five ions bound and demonstrate that a constriction near the intracellular end of the pore, acting as a gasket, prevents K+ ions from bypassing the blocker. This heretofore unrecognized 'gasket' ensures that the blocker can effectively displace K+ ions across the selectivity filter to generate exceedingly strong voltage sensitivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hille, B. Ion Channels of Excitable Membranes 3rd ed. (Sinauer, Sunderland, Massachusetts, USA, 2001).
Katz, B. Les constantes électriques de la membrane du muscle. Arch. Sci. Physiol. (Paris) 3, 285–299 (1949).
Noble, D. Electrical properties of cardiac muscle attributable to inward going (anomalous) rectification. J. Cell. Comp. Physiol. 66, 127–136 (1965).
Lopatin, A.N., Makhina, E.N. & Nichols, C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 (1994).
Ficker, E., Taglialatela, M., Wible, B.A., Henley, C.M. & Brown, A.M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science 266, 1068–1072 (1994).
Fakler, B. et al. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell 80, 149–154 (1995).
Woodhull, A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61, 687–708 (1973).
Guo, D., Ramu, Y., Klem, A.M. & Lu, Z. Mechanism of rectification in inward-rectifier K+ channels. J. Gen. Physiol. 121, 261–275 (2003).
Shin, H.G. & Lu, Z. Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine. J. Gen. Physiol. 125, 413–426 (2005).
Armstrong, C.M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58, 413–437 (1971).
Spassova, M. & Lu, Z. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J. Gen. Physiol. 112, 211–221 (1998).
Kuo, A. et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926 (2003).
Nishida, M., Cadene, M., Chait, B.T. & MacKinnon, R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 26, 4005–4015 (2007).
Nishida, M. & MacKinnon, R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell 111, 957–965 (2002).
Pegan, S. et al. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat. Neurosci. 8, 279–287 (2005).
Yang, J., Jan, Y.N. & Jan, L.Y. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron 14, 1047–1054 (1995).
Taglialatela, M., Ficker, E., Wible, B.A. & Brown, A.M. C-terminus determinants for Mg2+ and polyamine block of the inward rectifier K+ channel IRK1. EMBO J. 14, 5532–5541 (1995).
Guo, D. & Lu, Z. Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels. J. Gen. Physiol. 122, 485–500 (2003).
Harding, M.M. Small revisions to predicted distances around metal sites in proteins. Acta Crystallogr. D Biol. Crystallogr. 62, 678–682 (2006).
Lopatin, A.N., Makhina, E.N. & Nichols, C.G. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J. Gen. Physiol. 106, 923–955 (1995).
Shin, H.G., Xu, Y. & Lu, Z. Evidence for sequential ion-binding loci along the inner pore of the IRK1 inward-rectifier K+ channel. J. Gen. Physiol. 126, 123–135 (2005).
Stanfield, P.R. et al. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J. Physiol. (Lond.) 478, 1–6 (1994).
Lu, Z. & MacKinnon, R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature 371, 243–246 (1994).
Wible, B.A., Taglialatela, M., Ficker, E. & Brown, A.M. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature 371, 246–249 (1994).
Kubo, Y. & Murata, Y. Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel. J. Physiol. (Lond.) 531, 645–660 (2001).
Stampe, P., Arreola, J., Perez-Cornejo, P. & Begenisich, T. Nonindependent K+ movement through the pore in IRK1 potassium channels. J. Gen. Physiol. 112, 475–484 (1998).
French, R.J. & Shoukimas, J.J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys. J. 34, 271–291 (1981).
Yellen, G., Jurman, M.E., Abramson, T. & MacKinnon, R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science 251, 939–942 (1991).
Choi, K.L., Mossman, C., Aube, J. & Yellen, G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron 10, 533–541 (1993).
Holmgren, M., Smith, P.L. & Yellen, G. Trapping of organic blockers by closing of voltage-dependent K+ channels: evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109, 527–535 (1997).
Zhou, M., Morais-Cabral, J.H., Mann, S. & MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature 411, 657–661 (2001).
Guo, D. & Lu, Z. Mechanism of IRK1 channel block by intracellular polyamines. J. Gen. Physiol. 115, 799–814 (2000).
Kurata, H.T. et al. Molecular basis of inward rectification: polyamine interaction sites located by combined channel and ligand mutagenesis. J. Gen. Physiol. 124, 541–554 (2004).
Kurata, H.T., Marton, L.J. & Nichols, C.G. The polyamine binding site in inward rectifier K+ channels. J. Gen. Physiol. 127, 467–480 (2006).
Heginbotham, L. & MacKinnon, R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron 8, 483–491 (1992).
Kavanaugh, M.P. et al. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron 8, 493–497 (1992).
Lenaeus, M.J., Vamvouka, M., Focia, P.J. & Gross, A. Structural basis of TEA blockade in a model potassium channel. Nat. Struct. Mol. Biol. 12, 454–459 (2005).
Ahern, C.A., Eastwood, A.L., Lester, H.A., Dougherty, D.A. & Horn, R. A cation-pi interaction between extracellular TEA and an aromatic residue in potassium channels. J. Gen. Physiol. 128, 649–657 (2006).
Burley, S.K. & Petsko, G.A. Amino-aromatic interactions in proteins. FEBS Lett. 203, 139–143 (1986).
Bremi, T., Ernst, M. & Ernst, R.R. Side-chain motion with two degrees of freedom in peptides. An NMR study of phenylalanine side chains in antamanide. J. Phys. Chem. 98, 9322–9334 (1994).
Hodgkin, A.L. & Horowicz, P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J. Physiol. (Lond.) 148, 127–160 (1959).
Hagiwara, S. & Takahashi, K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J. Membr. Biol. 18, 61–80 (1974).
Leech, C.A. & Stanfield, P.R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J. Physiol. (Lond.) 319, 295–309 (1981).
Hagiwara, S. & Yoshii, M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J. Physiol. (Lond.) 292, 251–265 (1979).
Lu, Z. Mechanism of rectification in inward-rectifier K+ channels. Annu. Rev. Physiol. 66, 103–129 (2004).
Kubo, Y., Baldwin, T.J., Jan, Y.N. & Jan, L.Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362, 127–133 (1993).
Liman, E.R., Tytgat, J. & Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 (1992).
Kubo, Y., Reuveny, E., Slesinger, P.A., Jan, Y.N. & Jan, L.Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364, 802–806 (1993).
Dascal, N. et al. Expression of an atrial G-protein-activated potassium channel in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 90, 6596–6600 (1993).
Guo, D. & Lu, Z. Pore block versus intrinsic gating in the mechanism of inward rectification in strongly rectifying IRK1 channels. J. Gen. Physiol. 116, 561–568 (2000).
Guo, D. & Lu, Z. IRK1 inward rectifier K+ channels exhibit no intrinsic rectification. J. Gen. Physiol. 120, 539–551 (2002).
Huang, C.L., Feng, S. & Hilgemann, D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391, 803–806 (1998).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (2004).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Crystallographica Section D Biol. Crystallogr. 60, 2126–2132 (2004).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Laskowski, R.A., MacArthur, M.W., Moss, D. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Acknowledgements
We thank L. Jan (University of California, San Francisco) for sharing Kir2.1 and Kir3.1 cDNAs, J. Yang (Columbia University) for Kir2.1 cDNA in the pGEM-Hess vector; R. MacKinnon (Rockefeller University) for Kir3.1 cytoplasmic pore cDNA construct and for teaching molecular replacement method to Z.L.; K. Baek, R. Dominguez, K. Ferguson, S. Lee, S. Li, K. Schmitz and Y. Zhou for technical assistance and/or discussion, staff at CHESS A1 and NSLS X25 for technical service; and P. De Weer for review and discussion of our manuscript. This study was supported by a grant (GM55560) from the US National Institutes of Health and by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Y.X., H.-G.S. and Z.L. performed the experiments; Y.X., H.-G.S., S.S. and Z.L. analyzed the data; Y.X. and Z.L. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Table 1 (PDF 185 kb)
Rights and permissions
About this article
Cite this article
Xu, Y., Shin, HG., Szép, S. et al. Physical determinants of strong voltage sensitivity of K+ channel block. Nat Struct Mol Biol 16, 1252–1258 (2009). https://doi.org/10.1038/nsmb.1717
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1717
This article is cited by
-
Structure of potassium channels
Cellular and Molecular Life Sciences (2015)
-
The bundle crossing region is responsible for the inwardly rectifying internal spermine block of the Kir2.1 channel
Pflügers Archiv - European Journal of Physiology (2014)
-
Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease
Nature Reviews Neuroscience (2010)