Abstract
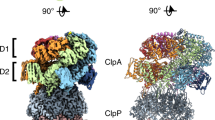
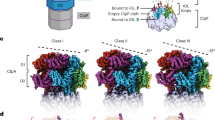
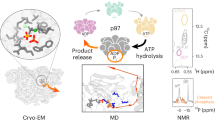
In the Escherichia coli ClpXP protease, a hexameric ClpX ring couples ATP binding and hydrolysis to mechanical protein unfolding and translocation into the ClpP degradation chamber. Rigid-body packing between the small AAA+ domain of each ClpX subunit and the large AAA+ domain of its neighbor stabilizes the hexamer. By connecting the parts of each rigid-body unit with disulfide bonds or linkers, we created covalently closed rings that retained robust activity. A single-residue insertion in the hinge that connects the large and small AAA+ domains and forms part of the nucleotide-binding site uncoupled ATP hydrolysis from productive unfolding. We propose that ATP hydrolysis drives changes in the conformation of one hinge and its flanking domains and that the changes are propagated around the AAA+ ring through the topologically constrained set of rigid-body units and hinges to produce coupled ring motions that power substrate unfolding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanson, P.I. & Whiteheart, S.W. AAA+ proteins: have engines, will work. Nat. Rev. Mol. Cell Biol. 6, 519–529 (2005).
White, S.R. & Lauring, B. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic 8, 1657–1667 (2007).
Baker, T.A. & Sauer, R.T. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim. Biophys. Acta 1823, 15–28 (2012).
Kim, D.Y. & Kim, K.K. Crystal structure of ClpX molecular chaperone from Helicobacter pylori. J. Biol. Chem. 278, 50664–50670 (2003).
Glynn, S.E., Martin, A., Nager, A.R., Baker, T.A. & Sauer, R.T. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell 139, 744–756 (2009).
Jeruzalmi, D., O'Donnell, M. & Kuriyan, J. Crystal structure of the processivity clamp loader gamma (γ) complex of E. coli DNA polymerase III. Cell 106, 429–441 (2001).
Skordalakes, E. & Berger, J.M. Structure of the Rho transcription terminator: mechanism of mRNA recognition and helicase loading. Cell 114, 135–146 (2003).
Erzberger, J.P., Mott, M.L. & Berger, J.M. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat. Struct. Mol. Biol. 13, 676–683 (2006).
Mott, M.L., Erzberger, J.P., Coons, M.M. & Berger, J.M. Structural synergy and molecular crosstalk between bacterial helicase loaders and replication initiators. Cell 135, 623–634 (2008).
Simonetta, K.R. et al. The mechanism of ATP-dependent primer-template recognition by a clamp loader complex. Cell 137, 659–671 (2009).
Singh, S.K. et al. Functional domains of the ClpA and ClpX molecular chaperones identified by limited proteolysis and deletion analysis. J. Biol. Chem. 276, 29420–29429 (2001).
Wojtyra, U.A., Thibault, G., Tuite, A. & Houry, W.A. The N-terminal zinc binding domain of ClpX is a dimerization domain that modulates the chaperone function. J. Biol. Chem. 278, 48981–48990 (2003).
Ortega, J. et al. ATPases bind simultaneously to opposite ends of ClpP peptidase to form active hybrid complexes. J. Struct. Biol. 146, 217–226 (2004).
Siddiqui, S.M., Sauer, R.T. & Baker, T.A. Role of the processing pore of the ClpX AAA+ ATPase in the recognition and engagement of specific protein substrates. Genes Dev. 18, 369–374 (2004).
Martin, A., Baker, T.A. & Sauer, R.T. Rebuilt AAA + motors reveal operating principles for ATP-fuelled machines. Nature 437, 1115–1120 (2005).
Martin, A., Baker, T.A. & Sauer, R.T. Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nat. Struct. Mol. Biol. 15, 1147–1151 (2008).
Martin, A., Baker, T.A. & Sauer, R.T. Diverse pore loops of the AAA+ ClpX machine mediate unassisted and adaptor-dependent recognition of ssrA-tagged substrates. Mol. Cell 29, 441–450 (2008).
Hersch, G.L., Burton, R.E., Bolon, D.N., Baker, T.A. & Sauer, R.T. Asymmetric interactions of ATP with the AAA+ ClpX6 unfoldase: allosteric control of a protein machine. Cell 121, 1017–1027 (2005).
Martin, A., Baker, T.A. & Sauer, R.T. Distinct static and dynamic interactions control ATPase-peptidase communication in a AAA+ protease. Mol. Cell 27, 41–52 (2007).
Kenniston, J.A., Baker, T.A., Fernandez, J.M. & Sauer, R.T. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell 114, 511–520 (2003).
Kim, Y.I., Burton, R.E., Burton, B.M., Sauer, R.T. & Baker, T.A. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol. Cell 5, 639–648 (2000).
Martin, A., Baker, T.A. & Sauer, R.T. Protein unfolding by a AAA+ protease: critical dependence on ATP-hydrolysis rates and energy landscapes. Nat. Struct. Mol. Biol. 15, 139–145 (2008).
Nager, A.R., Baker, T.A. & Sauer, R.T. Stepwise unfolding of a β-barrel protein by the AAA+ ClpXP protease. J. Mol. Biol. 413, 4–16 (2011).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Burton, R.E., Siddiqui, S.M., Kim, Y.I., Baker, T.A. & Sauer, R.T. Effects of protein stability and structure on substrate processing by the ClpXP unfolding and degradation machine. EMBO J. 20, 3092–3100 (2001).
Hoskins, J.R., Yanagihara, K., Mizuuchi, K. & Wickner, S. ClpAP and ClpXP degrade proteins with tags located in the interior of the primary sequence. Proc. Natl. Acad. Sci. USA 99, 11037–11042 (2002).
Bolon, D.N., Grant, R.A., Baker, T.A. & Sauer, R.T. Nucleotide-dependent substrate handoff from the SspB adaptor to the AAA+ ClpXP protease. Mol. Cell 16, 343–350 (2004).
Kenniston, J.A., Baker, T.A. & Sauer, R.T. Partitioning between unfolding and release of native domains during ClpXP degradation determines substrate selectivity and partial processing. Proc. Natl. Acad. Sci. USA 102, 1390–1395 (2005).
Aubin-Tam, M.E., Olivares, A.O., Sauer, R.T., Baker, T.A. & Lang, M.J. Single-molecule protein unfolding and translocation by an ATP-fueled proteolytic machine. Cell 145, 257–267 (2011).
Bochtler, M. et al. The structures of HslU and the ATP-dependent protease HslU-HsIV. Nature 403, 800–805 (2000).
Sousa, M.C. et al. Crystal and solution structures of an HslUV protease-chaperone complex. Cell 103, 633–643 (2000).
Guo, F., Maurizi, M.R., Esser, L. & Xia, D. Crystal structure of ClpA, an Hsp100 chaperone and regulator of ClpAP protease. J. Biol. Chem. 277, 46743–46752 (2002).
Krzywda, S. et al. The crystal structure of the AAA domain of the ATP-dependent protease FtsH of Escherichia coli at 1.5 Å resolution. Structure 10, 1073–1083 (2002).
Cha, S.S. et al. Crystal structure of Lon protease: molecular architecture of gated entry to a sequestered degradation chamber. EMBO J. 29, 3520–3530 (2010).
Duman, R.E. & Löwe, J. Crystal structures of Bacillus subtilis Lon protease. J. Mol. Biol. 401, 653–670 (2010).
Wang, F. et al. Structure and mechanism of the hexameric MecA-ClpC molecular machine. Nature 471, 331–335 (2011).
Joshi, S.A., Baker, T.A. & Sauer, R.T. C-terminal domain mutations in ClpX uncouple substrate binding from an engagement step required for unfolding. Mol. Microbiol. 48, 67–76 (2003).
Joshi, S.A., Hersch, G.L., Baker, T.A. & Sauer, R.T. Communication between ClpX and ClpP during substrate processing and degradation. Nat. Struct. Mol. Biol. 11, 404–411 (2004).
Crooks, G.E., Hon, G., Chandonia, J.M. & Brenner, S.E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Dombkowski, A.A. Disulfide by Design™: a computational method for the rational design of disulfide bonds in proteins. Bioinformatics 19, 1852–1853 (2003).
Nørby, J.G. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 156, 116–119 (1988).
Levchenko, I., Seidel, M., Sauer, R.T. & Baker, T.A. A specificity-enhancing factor for the ClpXP degradation machine. Science 289, 2354–2356 (2000).
Acknowledgements
This work was supported by US National Institutes of Health grant AI-15706 (R.T.S.). T.A.B. is an employee of the Howard Hughes Medical Institute. We thank B. Stinson and B. Sosa for help and discussions and P. Schwille (Dresden University of Technology) for providing the Kaede plasmid.
Author information
Authors and Affiliations
Contributions
S.E.G. and A.R.N. conducted all experiments. S.E.G., A.R.N., T.A.B. and R.T.S. contributed to experimental design, data interpretation and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Glynn, S., Nager, A., Baker, T. et al. Dynamic and static components power unfolding in topologically closed rings of a AAA+ proteolytic machine. Nat Struct Mol Biol 19, 616–622 (2012). https://doi.org/10.1038/nsmb.2288
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2288
This article is cited by
-
Coupling of distant ATPase domains in the circadian clock protein KaiC
Nature Structural & Molecular Biology (2022)
-
Azlocillin can be the potential drug candidate against drug-tolerant Borrelia burgdorferi sensu stricto JLB31
Scientific Reports (2020)
-
Mechanistic insight into TRIP13-catalyzed Mad2 structural transition and spindle checkpoint silencing
Nature Communications (2017)
-
Assessing heterogeneity in oligomeric AAA+ machines
Cellular and Molecular Life Sciences (2017)
-
Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines
Nature Reviews Microbiology (2016)