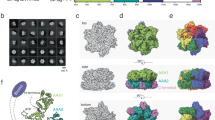
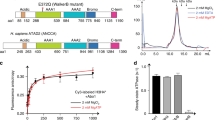
Abstract
Human HIRA, ASF1a, ASF1b and CAF-1 are evolutionally conserved histone chaperones that form multiple functionally distinct chromatin-assembly complexes, with roles linked to diverse nuclear process, such as DNA replication and formation of heterochromatin in senescent cells. We report the crystal structure of an ASF1a–HIRA heterodimer and a biochemical dissection of ASF1a's mutually exclusive interactions with HIRA and the p60 subunit of CAF-1. The HIRA B domain forms an antiparallel β-hairpin that binds perpendicular to the strands of the β-sandwich of ASF1a, via β-sheet, salt bridge and van der Waals contacts. The N- and C-terminal regions of ASF1a and ASF1b determine the different affinities of these two proteins for HIRA, by contacting regions outside the HIRA B domain. CAF-1 p60 also uses B domain–like motifs for binding to ASF1a, thereby competing with HIRA. Together, these studies begin to define the molecular determinants of assembly of functionally diverse macromolecular histone chaperone complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Loyola, A. & Almouzni, G. Histone chaperones, a supporting role in the limelight. Biochim. Biophys. Acta 1677, 3–11 (2004).
Smith, S. & Stillman, B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58, 15–25 (1989).
Kaufman, P.D., Kobayashi, R. & Stillman, B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 11, 345–357 (1997).
Linger, J. & Tyler, J.K. The yeast histone chaperone chromatin assembly factor 1 protects against double-strand DNA-damaging agents. Genetics 171, 1513–1522 (2005).
Myung, K., Pennaneach, V., Kats, E.S. & Kolodner, R.D. Saccharomyces cerevisiae chromatin-assembly factors that act during DNA replication function in the maintenance of genome stability. Proc. Natl Acad. Sci. USA 100, 6640–6645 (2003).
Gaillard, P.H. et al. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell 86, 887–896 (1996).
Moggs, J.G. et al. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol. Cell. Biol. 20, 1206–1218 (2000).
Green, C.M. & Almouzni, G. Local action of the chromatin assembly factor CAF-1 at sites of nucleotide excision repair in vivo. EMBO J. 22, 5163–5174 (2003).
Enomoto, S., McCune-Zierath, P.D., Gerami-Nejad, M., Sanders, M.A. & Berman, J. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 11, 358–370 (1997).
Enomoto, S. & Berman, J. Chromatin assembly factor I contributes to the maintenance, but not the re-establishment, of silencing at the yeast silent mating loci. Genes Dev. 12, 219–232 (1998).
Monson, E.K., de Bruin, D. & Zakian, V.A. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc. Natl Acad. Sci. USA 94, 13081–13086 (1997).
Kaya, H. et al. FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104, 131–142 (2001).
Sharp, J.A., Franco, A.A., Osley, M.A. & Kaufman, P.D. Chromatin assembly factor I and Hir proteins contribute to building functional kinetochores in S. cerevisiae. Genes Dev. 16, 85–100 (2002).
Kaufman, P.D., Cohen, J.L. & Osley, M.A. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol. Cell. Biol. 18, 4793–4806 (1998).
Phelps-Durr, T.L., Thomas, J., Vahab, P. & Timmermans, M.C. Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell 17, 2886–2898 (2005).
Sharp, J.A., Fouts, E.T., Krawitz, D.C. & Kaufman, P.D. Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol. 11, 463–473 (2001).
Sherwood, P.W., Tsang, S.V. & Osley, M.A. Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 28–38 (1993).
Zhang, R. et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005).
Ray-Gallet, D. et al. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol. Cell 9, 1091–1100 (2002).
Tagami, H., Ray-Gallet, D., Almouzni, G. & Nakatani, Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61 (2004).
Loppin, B. et al. The histone H3.3 chaperone HIRA is essential for chromatin assembly in the male pronucleus. Nature 437, 1386–1390 (2005).
Sutton, A., Bucaria, J., Osley, M.A. & Sternglanz, R. Yeast ASF1 protein is required for cell cycle regulation of histone gene transcription. Genetics 158, 587–596 (2001).
Tyler, J.K. et al. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402, 555–560 (1999).
Sillje, H.H. & Nigg, E.A. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr. Biol. 11, 1068–1073 (2001).
Tyler, J.K. et al. Interaction between the Drosophila CAF-1 and ASF1 chromatin assembly factors. Mol. Cell. Biol. 21, 6574–6584 (2001).
Mello, J.A. et al. Human Asf1 and CAF-1 interact and synergize in a repair-coupled nucleosome assembly pathway. EMBO Rep. 3, 329–334 (2002).
Krawitz, D.C., Kama, T. & Kaufman, P.D. Chromatin assembly factor I mutants defective for PCNA binding require Asf1/Hir proteins for silencing. Mol. Cell. Biol. 22, 614–625 (2002).
Schulz, L.L. & Tyler, J.K. The histone chaperone ASF1 localizes to active DNA replication forks to mediate efficient DNA replication. FASEB J. 20, 488–490 (2006).
Franco, A.A., Lam, W.M., Burgers, P.M. & Kaufman, P.D. Histone deposition protein Asf1 maintains DNA replisome integrity and interacts with replication factor C. Genes Dev. 19, 1365–1375 (2005).
Munakata, T., Adachi, N., Yokoyama, N., Kuzuhara, T. & Horikoshi, M. A human homologue of yeast anti-silencing factor has histone chaperone activity. Genes Cells 5, 221–233 (2000).
Mousson, F. et al. Structural basis for the interaction of Asf1 with histone H3 and its functional implications. Proc. Natl Acad. Sci. USA 102, 5975–5980 (2005).
Kirov, N., Shtilbans, A. & Rushlow, C. Isolation and characterization of a new gene encoding a member of the HIRA family of proteins from Drosophila melanogaster. Gene 212, 323–332 (1998).
Nelson, D.M. et al. Coupling of DNA synthesis and histone synthesis in S phase independent of cyclin/cdk2 activity. Mol. Cell. Biol. 22, 7459–7472 (2002).
Daganzo, S.M. et al. Structure and function of the conserved core of histone deposition protein Asf1. Curr. Biol. 13, 2148–2158 (2003).
Sanematsu, F. et al. Asf1 is required for viability and chromatin assembly during DNA replication in vertebrate cells. J. Biol. Chem. 281, 13817–13827 (2006).
Zhao, K., Harshaw, R., Chai, X. & Marmorstein, R. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases. Proc. Natl Acad. Sci. USA 101, 8563–8568 (2004).
Adams, P.D. et al. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 16, 6623–6633 (1996).
McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 (2005).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Nicholls, A., Sharp, K. & Honig, B. GRASP-graphical representation and analysis of surface properties. Proteins Struct. Funct. Genet. 11, 281ff (2003).
Acknowledgements
We thank M. Allaire for assistance with data collection. This work was supported by US National Institutes of Health grants to R.M. and P.D.A., a Leukemia and Lymphoma Society Scholar Award to P.D.A. and a grant from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health, awarded to the Wistar Institute. Part of this research was conducted on beamline X6A at the National Synchrotron Light Source at Brookhaven National Laboratory, which is supported by the US Department of Energy under contract no. DE-AC02-98CH10886. Beamline X6A is funded by the US National Institutes of Health, National Institute of General Medical Sciences, under agreement Y1 GM-0080-03.
Author information
Authors and Affiliations
Contributions
Y.T. and M.V.P. designed and carried out the reported experiments and prepared manuscript figures and text; K.Z., M.G., A.C. and R.D. carried out preliminary studies that led to the reported experiments; P.D.A and R.M. designed and supervised experiments and prepared manuscript text. All authors read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Sequence alignment of ASF1 homologs. (PDF 2466 kb)
Supplementary Fig. 2
ASF1a binding to HIRA deletion constructs. (PDF 558 kb)
Rights and permissions
About this article
Cite this article
Tang, Y., Poustovoitov, M., Zhao, K. et al. Structure of a human ASF1a–HIRA complex and insights into specificity of histone chaperone complex assembly. Nat Struct Mol Biol 13, 921–929 (2006). https://doi.org/10.1038/nsmb1147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1147
This article is cited by
-
Molecular complex detection in protein interaction networks through reinforcement learning
BMC Bioinformatics (2023)
-
Chromatin balances cell redox and energy homeostasis
Epigenetics & Chromatin (2023)
-
Histone chaperone ASF1 mediates H3.3-H4 deposition in Arabidopsis
Nature Communications (2022)
-
Tousled-like kinase 2 targets ASF1 histone chaperones through client mimicry
Nature Communications (2022)
-
Distinct role of histone chaperone Asf1a and Asf1b during fertilization and pre-implantation embryonic development in mice
Epigenetics & Chromatin (2021)